Understanding The Genomics Of Non
Advances in whole genome sequencing technology have vastly improved our insights into the complexity and heterogeneity of bladder cancer. In particular, parallel efforts to comprehensively characterize the molecular composition of muscle-invasive disease have revealed that MIBC can be broadly grouped into basal and luminal subtypes that are similar to those found in breast cancer, with distinct clinical features.1214 Efforts to similarly characterize NMIBC have also been undertaken, with mutations identified in the TERT promoter,15 FGFR3,1618 PIK3CA,19 and STAG220. Hurst and colleagues described two genomic subtypes for stage Ta tumors one group was characterized by no detectable copy-number alterations, while the second group was defined by a loss of chromosome 9q.21
Gene Therapy Promising In Bcg
Sara Freeman
More than half the patients with high-grade bacillus Calmette-Guérin -unresponsive nonmuscle invasive bladder cancer treated with nadofaragene firadenovec, an investigational intravesical viral gene therapy, achieved a clinical response at 3 months in a phase 3 trial.
The results “provide a significant efficacy benefit that, pending regulatory approval, might offer patients with a difficult-to-treat bladder cancer a bladder-sparing alternative,” said Neal Shore, MD, medical director for the Carolina Urologic Research Center in Myrtle Beach, South Carolina.
There is clearly an unmet need for new bladder-sparing treatments in these patients, said Fred Witjes, MD, from the Radboud Institute for Molecular Life Science in Nijmegen, the Netherlands, who discussed trial findings during the virtual European Association of Urology 2020 Congress.
“The drugs that we have are old and there is a limited availability for both MMC and BCG. We need some alternatives for initial adjuvant therapy,” he explained. “The unmet need is, of course, especially there in BCG-unresponsive patients or BCG-unresponsive CIS .”
“Clinically appropriate patients with BCG-unresponsive NMIBC are currently faced with radical cystectomy,” Shore explained during his presentation at the congress.
“Follow-up and treatment of these patients is ongoing in an extension study,” Shore said.
European Association of Urology 2020 Congress. Presented July 17, 2020.
Priming The Bladder For Intravesical Gene Therapy
The urothelium of the bladder is a complex, multilayer surface that is tasked with providing a barrier from pathogens and urinary waste products.35 The superficial part of surface is also known as the GAG layer and is comprised of a hydrophilic, polyanionic barrier of glycoproteins and proteoglycans. The main components of the GAG layer include chondroitin sulfate and hyaluronic acid, and these in part work to provide protection against the unwanted internalization of ions, solutes, water, and urinary bacteria that may be present in excreted urine.35 Dysfunction of the GAG layer has been implicated in interstitial cystitis and the development of chronic pelvic pain syndrome.35
Successful intravesical gene therapy requires a means to permeate the GAG layer such that the underlying urothelium can be accessed for efficient viral transduction. As noted earlier, the non-ionic detergent Big CHAP was an early transduction-promoting agent used in initial intravesical adenoviral vector clinical studies, described first by Connor and colleagues.33 Further investigation of this agent led to the discovery of Syn3, a polyamide compound demonstrated to improve intravesical gene delivery.36 This compound would prove to be essential in overcoming the intravesical gene transduction limitations encountered in previous clinical trials.
Recommended Reading: What Antibiotic Do You Take For A Bladder Infection
Gene Therapy In Treating Patients With Advanced Bladder Cancer
| The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government. Read our disclaimer for details. |
| First Posted : November 25, 2003Last Update Posted : January 23, 2013 |
| Intervention/treatment | Phase |
|---|---|
| Recurrent Bladder CancerStage I Bladder CancerStage II Bladder CancerStage III Bladder CancerStage IV Bladder CancerTransitional Cell Carcinoma of the Bladder | Biological: Ad5CMV-p53 gene |
PRIMARY OBJECTIVES:
I. Determine the safety and toxicity of adenovirus p53 gene therapy in patients with locally advanced or metastatic bladder cancer.
II. Measure infection with Ad-p53 and confirm expression of p53 after infection.
III. Characterize clinical response of measurable tumor in these patients. IV. Determine the duration of effect of this treatment in these patients. V. Define the time course of elimination of vector from urinary bladder.
OUTLINE: This is a dose escalation study.
Group 1 patients receive adenovirus p53 intravesically on days 1 and 4. Treatment continues every 4 weeks for a maximum of 6 courses in the absence of disease progression or unacceptable toxicity.
Patients are followed on day 28, then every 3 months for 1 year or until disease progression.
Targeted Therapy Drugs For Bladder Cancer
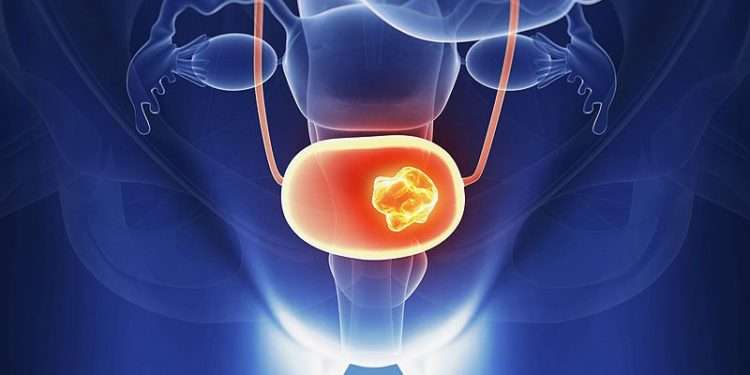
As researchers have learned more about the changes inside cells that cause cancer, they have developed newer drugs that target some of these changes. These targeted drugs work differently from other types of treatment, such as chemotherapy . They may work in some cases when other treatments don’t. Targeted drugs also often have different types of side effects.
Also Check: Can Stress And Anxiety Cause Overactive Bladder
Treating Bladder Cancer: Engineering Of Current And Next Generation Antibody
- 1Institute for Organic Chemistry and Biochemistry, Technical University of Darmstadt, Darmstadt, Germany
- 2Ferring Darmstadt Laboratory, Biologics Technology and Development, Darmstadt, Germany
- 3Ferring Pharmaceuticals, International PharmaScience Center, Copenhagen, Denmark
- 4Global Pharmaceutical Research and Development, Ferring International Center S.A., Saint-Prex, Switzerland
Gene Therapy Shows Encouraging Clinical Efficacy/safety In Bcg
A novel gene therapy is being investigated for the treatment of Bacillus Calmette-Guerin-unresponsive non-muscle invasive bladder cancer, and the preliminary findings signal a high response rate with little toxicity.
EG-70, a non-viral gene therapy, appears to be a safe and well-tolerated treatment for patients with Bacillus Calmette-Guerin -unresponsive non-muscle invasive bladder cancer , with promising clinical efficacy, according to phase 1/2 findings from the LEGEND trial, announced by enGene, Inc.1
“We are thrilled to announce the safety and clinical efficacy of EG-70 in patients with BCG-unresponsive NMIBC who are facing bladder removal as an alternative,” said Jason Hanson, chief executive officer at enGene, in a press release. “EG-70 was designed ab initio as an intravesical monotherapy that would slot directly into current medical practice to provide ease-of-use and increased access to patients. With the initial results providing a clear best-in-class trajectory, we are confident that EG-70 will be the organ-sparing solution that is a game-changer for patients and physicians.”
Early results were reported for 6 patients with BCG-unresponsive NMIBC who had reached the 3-month efficacy assessment. Of those assessed, 5 patients had a CR to treatment with EG-70, achieving an 83% CR rate.
References:
You May Like: Can A Ct Urogram Detect Bladder Cancer
Th Esou: Gene Therapy Shows Promise In Non
Gene therapy shows promise in the treatment of non muscle invasive bladder cancer with current trials indicating that the gene therapy approach might offer less toxic side effects, according to Prof. Seth Lerner who spoke on the subject during the 9th EAU Section of Oncological Urology Meeting held this weekend in Hamburg, Germany.
Saying that non-muscle invasive urothelial bladder cancer has a high recurrence rate and no new drug approvals in recent years, Lerner said there is a need to consider other treatment options aside from chemotherapy and targeted therapeutics.
Moreover, he said that NMIBC is the most expensive cancer from diagnosis until death and is linked with a long-term progression rate of 30 to 40% despite high initial response rates to induction BCG with maintenance. Among the emerging therapies is gene therapy, which Lerner said have attracted several extensive studies in recent years.
Bladder cancer is an ideal target for gene therapy due to the ease of access to tumors with opportunities for intravesical therapy as well as direct tumor injection, he said. In gene therapy a vector or delivery module is required and two current methods are the so-called viral and non-viral mediated gene delivery.
Gene therapys effects are achieved through gene augmentation or gene blocking, and Lerner noted that there are a number of gene therapies that have been developed such as corrective, immunomodulatory, suicide and complementary gene therapy.
The Rna Isoform Landscape Of Cancer
Cancers display widespread RNA dysregulation, including alternative splicing of transcripts. These aberrant transcripts are often translated into cancer-specific proteins and have been shown to affect cancer initiation, progression, metastasis, and drug resistance. While it has been recently reported that cancer-specific isoforms can be used as novel prognostic biomarkers and serve as an untapped source of drug targets for immuno-oncology, the vast majority of cancer specific isoforms remain unknown. With at least 95% of genes undergoing alternative splicing and producing multiple isoforms, much remains to be discovered.
Join us for this webinar to learn:
- The roles of RNA isoforms in cancer biology
- How long-read RNA sequencing can help you uncover the molecular heterogeneity of cancers
- Why the Iso-Seq method is the best way to identify RNA isoforms in cancer
“Quotation here”
Also Check: How Is Immunotherapy Administered For Bladder Cancer
Whats New In Bladder Cancer Research
Research on bladder cancer is taking place in many university hospitals, medical centers, and other institutions around the world. Each year, scientists find out more about what causes the disease, how to find it as soon as possible, and how to better treat it. Most experts agree that treatment in a clinical trial should be considered for any type or stage of bladder cancer. This way people can get the best treatment available now and may also get the new treatments that are thought to be even better. The new and promising treatments discussed here are only available in clinical trials.
Gene Therapy Appears Effective In Bladder Cancer Patients With Few Options
REPORTING FROM GUCS 2020
SAN FRANCISCO A novel gene therapy may expand treatment options for nonmuscle invasive bladder cancer that does not respond to Bacillus Calmette-Guerin , new research suggests.
Dr. Stephen A. Boorjian
The therapy, nadofaragene firadenovec, is a recombinant adenovirus that is instilled into the bladder and delivers the human interferon alpha-2b gene, leading to expression of the immune cytokine.
At 3 months, nadofaragene firadenovec had produced a complete response in 53.4% of patients with carcinoma in situ , and the rate of high-grade recurrence-free survival was 59.6% in all patients. Although most patients experienced adverse events in this trial, few had serious events .
Stephen A. Boorjian, MD, of the Mayo Clinic in Rochester, Minn., presented these results at the 2020 Genitourinary Cancers Symposium, sponsored by the American Society for Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
The optimal management for patients with BCG-unresponsive nonmuscle invasive bladder cancer remains to be established, Dr. Boorjian said. National and organizational guidelines recommend radical cystectomy in this setting, but we have to acknowledge that many of our patients will be either unwilling or unfit to undergo what is often a highly morbid operation.
Read Also: Bladder Cancer Drugs In Development
Additional Gene Therapy With Raav
Log in to MyKarger to check if you already have access to this content.
Buy a Karger Article Bundle and profit from a discount!
If you would like to redeem your KAB credit, please log in.
Save over 20%
- Rent for 48h to view
- Buy Cloud Access for unlimited viewing via different devices
- Synchronizing in the ReadCube Cloud
- Printing and saving restrictions apply
USD 8.50
- Access to all articles of the subscribed year guaranteed for 5 years
- Unlimited re-access via Subscriber Login or MyKarger
- Unrestricted printing, no saving restrictions for personal use
The final prices may differ from the prices shown due to specifics of VAT rules.
Study Design And Participants
The protocol and accrual timeline were designed by the Society of Urologic Oncology Clinical Trials Consortium. The study was done in accordance with the Declaration of Helsinki, in compliance with Good Clinical Practice Guidelines. The study protocol was approved by an institutional review board for each centre before accrual. The study procedures and analyses were done per protocol and all protocol amendments were approved by the institutional review board before implementation.
Read Also: Reasons For Bladder Control Loss
Immunogene Therapy Using Interferon
Immunogene therapy refers to the delivery of genetic material for the purpose of modulating a host immune response. In immunogene therapy, tumor-induced immunosuppression can be altered, and antigen-specific antitumor responses can be stimulated. Therapies such as high-dose IL-2 have been used effectively in treating renal cell carcinoma, albeit with significant systemic adverse events. Interferon- belongs to the family of cytokine proteins and works to pleiotropically impede tumor cell growth. IFN has been demonstrated to augment the response of T helper type 1 immune responses when combined with BCG, and as such its use as combination therapy with BCG has been explored in several trials. In a national multi-institutional phase II trial evaluate the combination of IFN and BCG in patients with non-muscle invasive disease, 59% of BCG-naïve and 45% of prior BCG unresponsive patients remained disease free at 24-months.37 A hypothetical way to potentiate the immunogene effect of IFN may be to deliver it within a gene therapy/adenoviral construct, ideally increasing transfection rate of IFN into urothelial cells and stimulating an immune response by virtue of the adenovirus vehicle itself . Unlike the delivery of systemic cytokines such as IL-2 therapy, intravesically delivered IFN has been established as well tolerated by patients and is likely well tolerated even through gene therapy.
Major Issues And Future Prospects For Gene Therapy
One major issue is the safety of gene therapy in terms of its impact on the environment as well as long term safety in patients. Schenk-Braat et al found that only half of all registered clinical trials included viral shedding data and what data was available was primarily for the time after virus delivery and not at the time of delivery. These questions should also be raised for plasmid based gene therapy. Long term follow-up data on patients who have received gene therapy may further ameliorate the safety concerns of this therapy. This will result in gene therapy being more readily applied to other non-malignant conditions. The development of better plasmids and ways to integrate plasmids into the chromosome may lead to the greater use of plasmid rather than viral vectors for urological gene therapy.
MicroRNAs are new targets for cancer therapy. These are small non-coding RNA molecules that bind to complementary sequences in the protein coding regions of mRNA and block their translation. Their expression levels vary in cancer and normal tissues . MiRNA-203 and MiRNA-221 have been shown to modulate the growth and apoptosis of human bladder cancer cell lines and these could be new targets for therapy. Given the function of miRNA it is possible that these molecules could also be targets for non-cancer related bladder dysfunctions. This has not yet been explored and identifying such molecules may improve our knowledge of the development of these conditions.
Also Check: How To Help A Weak Bladder Naturally
About Blackstone Life Sciences
FerGene, a new gene therapy company and Ferring subsidiary, has been created to potentially commercialize nadofaragene firadenovec in the U.S. and to advance the global clinical development. FerGenes goal is to bring this promising therapy to a patient population which has seen little improvement in their standard of care over the past twenty years. Blackstone Life Sciences will invest $400 million USD and Ferring will invest up to $170 million USD in FerGene.
Cancer Gene Therapy Backed By Blackstone Gets Trial Win
A gene therapy for bladder cancer that recently received $400 million in support from the private equity company Blackstone Group helped more than half of treated patients with resistant disease achieve remission.
The therapy, called nadofaragene firadenovec, was discovered by a Finnish-based research institute and first entered clinical study in 2012. The data revealed today at the Society of Urologic Oncology meeting came from a Phase 3 trial that is part of the agent’s Biologics License Application now before the FDA.
Licensed by its original owner, FKD Therapies Oy, to Switzerland-based Ferring Pharmaceuticals, nadofaragene firadenovec is now in the hands of the U.S. subsidiary FerGene. That company was created with the Blackstone investment and an additonal $170 million from Ferring. FerGene will commercialize the gene therapy in the U.S., with Ferring holding rights elsewhere.
Nadofaragene firadenovec is an an adenovirus-based gene therapy encoding production of the immunity-stimulating protein interferon alfa-2b. Viral vectors containing the gene are administered by catheter once every three months into the bladder, where they are absorbed into cells in the organ’s walls and begin stimulating interferon.
The clinical trial enrolled 157 patients with bladder cancer that has not spread to muscle walls and has stopped responding to treatment with Bacillus Calmette-Guérin vaccine.
Recommended Reading: Can I Have A Bladder Infection Without Pain
Managing Nmibc With Bcg
BCG was initially developed as a vaccine for tuberculosis, and it is a live attenuated drug derived from Mycobacterium bovis. The use of BCG for the treatment of cancer can be traced back to the observations of Pearl in 1929, when patients with a diagnosis of tuberculosis were noted to have lower rates of cancer at the time of their autopsies.3 In 1976, Morales and colleagues published the landmark study describing the first use of BCG to treat bladder cancer intravesically.4 The intravesical introduction of BCG causes an infection of urothelial and tumor cells through a fibronectin-mediated process.5 This, in turn, promotes a local immune response facilitated by granulocytes, macrophages, and T-helper cells. Anti-tumor responses result partly from the antigen-processing functions of these phagocytic cells. Several cytokines, including tumor necrosis factor- , interferon , and interleukin -1, IL-2, IL-6, IL-8, IL-10, IL-12, and IL-17 participate in the immune stimulation process as demonstrated by post-BCG urine analysis.6,7 More recently, the role of autophagy in generating an epigenetic reprogramming of monocytes has also been implicated in the mechanism of generating an intravesical BCG-stimulated host response.8