A Golden Age Of Bladder Cancer Drug Development
- References:
- Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507:315-22, 2014
- Sharma P, Retz M, Siefker-Radtke A, et al: Nivolumab in metastatic urothelial carcinoma after platinum therapy : a multicentre, single-arm, phase 2 trial. The Lancet Oncology 18:312-322, 2017
- Rosenberg JE, Sridhar SS, Zhang J, et al: Updated results from the enfortumab vedotin phase 1 study in patients with metastatic urothelial cancer . Journal of Clinical Oncology 36:4504-4504, 2018
- Siefker-Radtke AO, Necchi A, Park SH, et al: First results from the primary analysis population of the phase 2 study of erdafitinib in patients with metastatic or unresectable urothelial carcinoma and FGFR alterations . J Clin Oncol 36, 2018
Our Understanding Of Bladder Cancer
In Europe, bladder cancer is the 5th most common cancer and it is estimated that by the year 2030, around 219,000 people will be diagnosed across Europe each year.
People living with the disease often face poor outcomes, a 5% survival rate over five years for metastatic settings. At Janssen we are embracing this challenge.
There is strong scientific evidence to show that the earlier the diagnosis of bladder cancer, the better the prognosis is for patients.
Study Strengths And Limitations
The major strength of this study is the construction and validation of a prognostic signature based on lipid metabolism-related genes that is closely related to prognosis of BLCA patients. The main limitation of the study is the absence of experimental validation in vivo and vitro. Therefore, further experiments should be performed to validate the functions of lipid metabolism-related genes in BLCA.
Also Check: Botox For Overactive Bladder Cost
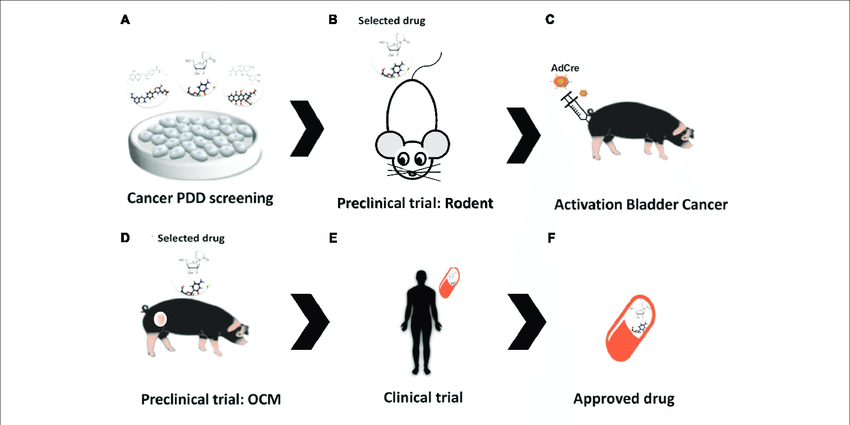
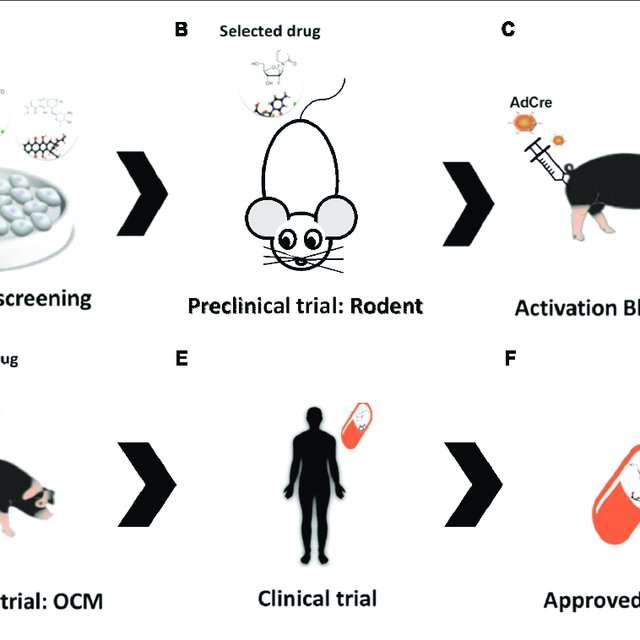
Moving Toward The Clinic
Each of the researchersâ survival studies of bladder cancer development or drug response took over a year for them to conduct. Hsieh noted that during this time, the field âexploded.â Several new classes of drugs have improved treatment options for bladder cancer patients. These include immunotherapies, including checkpoint inhibitors like Bavencio and antibody-drug conjugates that use immune proteins to concentrate cancer drugs in tumor tissue, as well as drugs that target a commonly overproduced protein. But many will still die after their disease spreads, and Hsieh and Jana hope that their work is a step toward offering patients another life-extending treatment.
âThe big question for me is, how does translation interface with these drugs?â Hsieh said.
They aim to test eFT508âs potential as a combination therapy with other bladder cancer treatments, starting with mouse models of bladder cancer. They also hope to tease out whether eFT508âs anti-tumor effects come from reducing protein synthesis in bladder tumor cells themselves, or from effects on other cell types.
Additionally, Jana is working to better understand how BBN treatment causes the increase in protein synthesis that facilitates tumor development, as well as untangling eIF4Eâs role in bladder tumor development and progression. Sheâs also extending her focus to other regulators of protein synthesis.
Biomarkers And Antibodies Development For Bladder Cancer
With years of experience in developing antibodies for analytical research and diagnostic applications, Creative Biolabs specializes in custom antibody and assay development for the diagnosis of a variety of diseases and infections. Particularly, we provide high-quality in vitro diagnostic antibody development services for the diagnosis of different cancers, such as bladder cancer.
Introduction of Bladder Cancer
Bladder cancer is the commonest malignancy of the urinary tract, accounting for 7 percent of all cancers in men and 2 percent of those in women. The main symptoms of bladder cancer include blood in the urine, pain with urination and low back pain. Although the accurate cause remains unclear, risk factors that may contribute to its development include cigarette smoking, occupational exposure to chemicals such as aniline dyes and aromatic amines, consumption of analgesics containing phenacetin, chronic infection or irritation of the bladder, and chemotherapeutic agents such as cyclophosphamide. Over 90% of malignant tumors of the bladder are ‘urothelial’ transitional cell carcinoma . Less common forms of bladder cancer include squamous cell carcinoma, adenocarcinoma, and neuroendocrine tumors. The grading and staging of bladder cancer use the TNM classification system, which provides important information for disease prognosis and prediction.
Fig 1. T-stages of transitional cell carcinoma bladder cancer .
Diagnosis of Bladder Cancer
Diagnostic Tumor Markers
You May Like: What Do Bladder Spasms Feel Like
Identification Of Potential Small Molecule Drugs
The connectivity Map database was used to predict potential drugs that may reverse or induce the biological states of BLCA based on the DELRGs. The DELRGs was submitted to the CMAP database to search small molecular drugs that could be used for BLCA treatment. The enrichment scores ranged from ââ1 to 1. A negative score suggested that the drug could be beneficial for BLCA treatment.
The Phase 2 Trial Helps Fine
The Phase 2 trial begins to gauge the effectiveness and tolerability of the treatment in patients with previously treated metastatic or unresectable urothelial carcinoma.
During our research, we also discovered an important biomarkerphosphate, a mineral found naturally in the body, adds De Porre. Patients who had a higher level of it had a better response to the treatment. This later becomes an important factor in determining effective dosage, which, after much work, the researchers zero in on by the end of 2014.
This class of agents has a very narrow therapeutic indextoo low or too high of a dose wont be effective and safe, says Kiran PatelKiran Patel,Vice President Clinical Development, Solid Tumor Franchise at Janssen, Vice President Clinical Development, Solid Tumor Franchise, Janssen.
Don’t Miss: Can A Bladder Infection Go Away On Its Own
New Drug Cuts The Risk Of Death In Bladder Cancer By 30% Compared With Chemotherapy Study Suggests
Shutterstock.com
A new type of drug that targets chemotherapy directly to cancer cells reduces the risk of death from the most common type of bladder cancer by 30%, a phase III trial in the New England Journal of Medicine has suggested.
Researchers at Queen Mary University of London and Barts Health NHS Trust set out to investigate the efficacy of an antibody-drug conjugate, enfortumab vedotin, in 608 adult patients with locally advanced or metastatic urothelial cancer, who had previously been treated with platinum-based chemotherapy and a PD-1 or PD-L1 inhibitor.
Globally, urothelial cancer accounts for around 549,000 new cases of bladder cancer and 200,000 deaths each year, and is generally treated with chemotherapy.
Unlike conventional chemotherapy treatments, which can damage healthy cells, antibody-drug conjugates are targeted medicines that deliver chemotherapy agents directly to the cancer cells.
In the study, half of the patients were randomly assigned to receive enfortumab vedotin and half to receive chemotherapy. The primary end point was overall survival and median follow-up was 11.1 months.
The researchers found that overall survival was longer in the enfortumab vedotin group than in the chemotherapy group .
Side effects were found to be manageable and similar to chemotherapy .
It reduced the death rate by 30% and beat chemotherapy in every setting, so this really is a big deal.
Promising In Clinical Trials
Fibroblast growth factor receptor signaling in bladder cancer has been investigated for two decades . However, more than 10 years later, antibodies and strategies to target the FGFR pathway were subsequently developed . Almost 10 years later, clinical trials on targeting this pathway in solid tumors are initiated and performed. In this section, we aim to discuss the current status of FGFR targeting in bladder cancer.
Read Also: What Are The Ingredients In Azo Bladder Control
Combination Of Chemotherapeutic Drug Mmc With Bcg/gemcitabine
BCG and Mitomycin C are representatives of clinical intravesical immunotherapy and chemotherapy drugs for NMIBC.
A randomized prospective trial involved 407 patients with intermediate- to high-risk NMIBC found that sequential combination of MMC plus BCG is more effective but more toxic than BCG alone. Thus, it was recommended to patients with a high likelihood of recurrence, such as those with recurrent T1 tumors . Another study including 151 patients with high-risk NMIBC demonstrated outstanding efficacy for sequential BCG and EMDA-MMC . The complete-response rate was 87%, with 86% and 93% remaining disease-free at one and two years respectively which is better than any previously published outcomes in this disease .
Gemcitabine is a pyrimidine analogue that incorporates into actively replicating DNA and thereby prevents further synthesis, whereas MMC cross-links DNA moieties to prevent synthesis . In addition, MMC is a vesicant to the urothelium, which could increase permeability to subsequent gemcitabine administration through its irritating action. So, it is available to combine MMC with gemcitabine as a possibly effective way to enhance mutual absorption and control tumor progression . Sequential intravesical gemcitabine and MMC in NMIBC patients appeared to be well tolerated and yielded a response in a good proportion of patients with recurrent BCG refractory bladder cancer or who are not surgical candidates .
Immunotherapy For Metastatic Urothelial Carcinoma Post
All five of the anti-PD-1 agents for urothelial carcinoma are currently approved by the FDA as treatment for LA/mUC patients who have disease progression during or following platinum-based chemotherapy or within 12 months of neoadjuvant or adjuvant treatment for localized disease with platinum-based chemotherapy. In the trials leading to their approval, ORRs across all patients ranged from 15% with atezolizumab in IMvigor210 to 21.1% with pembrolizumab in KEYNOTE-045. Median OS ranged from 6.5 months with avelumab to 18.2 months with durvalumab. Importantly, the phase III KEYNOTE-045 trial of pembrolizumab now has over 2 years of patient follow-up demonstrating a continued OS benefit over second-line chemotherapy with median OS of 10.1 months with pembrolizumab and 7.3 months with chemotherapy . The IMvigor211 study was a similarly designed phase III randomized trial comparing atezolizumab versus chemotherapy. However, the primary endpoint, OS, was tested hierarchically in prespecified populationsthat is, IC2/3 , followed by IC1/2/3, and then the intention-to-treat population. In the IC2/3 population, there was no significant difference in median OS , precluding further formal statistical analyses in the other prespecified populations and thereby resulting in an overall negative study.
Read Also: Why Does My Bladder Hurt When I Pee
New Treatments For Bladder Cancer In 2020
In 2019 and early 2020, the U.S. Food and Drug Administration approved a number of new drugs for bladder cancer of all stages, and more treatments are on the horizon. Heres a snapshot of whats happening right now in bladder cancer treatment:
Non-muscle invasive bladder cancer treatments
In patients with NMIBC, tumors are confined to the inner cell layer of the bladder and have not invaded the thick muscle tissue of the bladder. NMIBC is usually treated by surgical excision in a procedure known as trans urethral resection of bladder tumor , followed by repeated drug injections into the bladder most commonly with a drug called Bacillus Calmette-Guerin .
BCG is a bacterial vaccine that is primarily used to immunize against tuberculosis, but may also trigger a broader immune response against cancer cells. BCG certainly improves outcomes for patients whose tumors have been resected nevertheless, over a half of these patients will experience recurrence within one year, and some do not respond to BCG at all. Intravesical chemotherapy drugs are used for higher-grade NMIBC.
Newly emerging treatment options for NMIBC include:
Of course, the biggest news for NMIBC is the recent FDA approval of the immune checkpoint drug pembrolizumab for systemic treatment of high grade NMIBC that is not responsive to BCG. In clinical testing, 41% of patients experienced complete responses with median duration of 16 months.
Combination Of Parp Inhibitors With Immunotherapy
Epigenetics is defined as a heritable modification to DNA without alteration in the nucleotide sequence, resulting in altered gene transcription and chromatin structure. Epigenetic modifications include DNA methylation and post-translational histone modifications involving methylation or acetylation are common in bladder tumors. Growing evidence showed that epigenetic drugs, such as DNA methyltransferase inhibitors can upregulate immune signaling through demethylation of endogenous retroviruses and cancer testis antigens. It provides a strong rationale for the combination of epigenetic drugs with ICIs . Interestingly, RRx-001, not only a new DNA damage inducer, but also an epigenetic and immunomodulatory drug, has been recently investigated as single chemotherapeutic agent to re-sensitize tumor to prior therapy . The low toxicity profile of RRx-001 differentiates this agent from conventional anticancer drugs, such as chemotherapeutics, and epigenetic agents . Indeed, RRx-001 is able to trigger DNA damage response in urothelial bladder cancer cells, reducing the DNA methyltransferase1 levels and increasing the transcriptional levels of the interferon type III and the interferon stimulated genes . Thus, it enhances the sensitivity to ICIs. Criscuolo D et al. investigated the effects of combining three classes of drugs together with epigenetic agents and immune-checkpoint inhibitors in bladder cancer for the purpose of reducing toxicity and doses of monotherapy alone .
Don’t Miss: Why Do I Get Frequent Bladder Infections
A Surprising And Promising Discovery
Early on in the clinical trials, one of theJanssen clinical investigators races into Lorenzis office and shows him some exciting news on his phone: a picture of a recent full-body CT scan of a patient with metastatic bladder cancer.
We saw some promising activity in the patient after two cycles of therapy, Lorenzi recalls. We thought this could be the sign we were looking for, just not in the cancer we wereprimarily focused on. We were thrilled, but also mystified.
Once they did genetic testing of the tumor, they had clarity: The patient harbored a gene alteration, which likely controlled the growth of the tumor, Lorenzi explains.
After further research, the Janssen team pinpoints the specific gene mutations, and their frequency, that seem to respond most to the treatment. We learned during the course of our studies that bladder cancer patients with specific types ofalterations responded well to the drug, says Ademi Santiago-WalkerAdemi Santiago-Walker, Associate Director, Translational Research and Biomarker Lead at Janssen, Associate Director, Translational Research and Biomarker Lead, Janssen.
This was an especially exciting learning because, up until this time, these select mutations had traditionally been less responsive to many other treatments.
The Fda Grants The Treatment Breakthrough Therapy Designation
The FDA grants Breakthrough Therapy designation a process that expedites the development and review process for drugs that show significant promisefor the treatment.
This is based on data we had for 59 patients with metastatic cancer, all of whom had already failed one or more prior lines of treatment, De Porre explains. We found that 42% of people in this group were responders, which is very positive given the high unmet need in this population.”
Recommended Reading: Can Clindamycin Treat Bladder Infection
State Of Current Research
Table 1Full table
FGFR-targeting agents: B-701human immunoglobulin G1 monoclonal antibody directed against FGFR3 AZD4547selective FGFR1-3 tyrosine kinase inhibitor FPA144anti-FGFR2b humanized monoclonal antibody BAY1163877rogaratinib, selective FGFR1-3 tyrosine kinase inhibitor BGJ398selective FGFR1-3 tyrosine kinase inhibitor JNJ-42756493erdafitinib, selective pan-FGFR tyrosine kinase inhibitor LY3076226Antibody-Drug Conjugate . FGFR, fibroblast growth factor receptor.
Looking For More About The Latest Research
If you would like additional information about the latest areas of research regarding bladder cancer, explore these related items:
-
To find clinical trials specific to your diagnosis, talk with your doctor or search online clinical trial databases now.
-
Visit the Cancer.Net Blog to read reviews of recent research in bladder cancer and to listen to podcasts with expert perspectives on the topic.
-
Visit the website of Conquer Cancer, the ASCO Foundation, to find out how to help support cancer research. Please note that this link takes you to a separate ASCO website.
The next section in this guide is Coping with Treatment. It offers some guidance on how to cope with the physical, emotional, social, and financial changes that cancer and its treatment can bring. Use the menu to choose a different section to read in this guide.
Recommended Reading: What Can Help Overactive Bladder
If Treatment Does Not Work
Full recovery from bladder cancer is not always possible. If the cancer cannot be cured or controlled, the disease may be called advanced or metastatic.
This diagnosis is stressful, and for many people, advanced cancer is difficult to discuss. However, it is important to have open and honest conversations with your health care team to express your feelings, preferences, and concerns. The health care team has special skills, experience, expertise, and knowledge to support patients and their families, and is there to help. Making sure a person is physically comfortable, free from pain, and emotionally supported is extremely important.
Patients who have advanced cancer and who are expected to live less than 6 months may want to consider hospice care. Hospice care is a specific type of palliative care designed to provide the best possible quality of life for people who are near the end of life. You and your family are encouraged to talk with the health care team about hospice care options, which include hospice care at home, a special hospice center, or other health care locations. Nursing care and special equipment can make staying at home a workable option for many families. Learn more about advanced cancer care planning.
After the death of a loved one, many people need support to help them cope with the loss. Learn more about grief and loss.
The Power Of Targeted Transformation
We are focusing on better understanding the drivers of urothelial cancer, the most common type of bladder cancer and are exploring targeted therapies – alone and in combination – to transform outcomes. At Janssen Oncology, average isnt good enough. Were driven only to deliver the best outcomes to patients, partners, and the oncology community.
We see enormous potential in the use of companion diagnostics, to ensure patients have access to the latest and most appropriate therapeutic opportunities.
Recent research efforts in the field of precision oncology have provided key insights that have transformed the treatment paradigm, and will undoubtedly continue to expand our therapeutic options in the years to come.
Evidence suggests that the FGFR pathway may provide another option for treating bladder cancer. Current phase 3 trials are ongoing to investigate the use of FGFR inhibitors to treat FGFR3-altered metastatic urothelial carcinoma.
Recommended Reading: Doterra Oils For Bladder Infection
Combination Of Pi3k/akt/mtor Inhibitors With Immunotherapy Or Chemotherapy
The PI3K/AKT/mammalian target of rapamycin pathway is an important signal pathway closely related to protein synthesis, cell growth, survival and tumorigenesis . The deregulation of this signaling pathway is present in 42% of UC, including mutations, copy number alterations, or RNA expression changes . Despite the frequent deregulation, clinical trials using PI3K/mTOR inhibitors have not shown prominent success. The PIK3CA gene is an oncogene that implicates the overactivation of the PI3K/AKT/mTOR pathway. Recurrent somatic mutations of PIK3CA increase the activity of PI3Ks and the loss of phosphatase and tension homolog also can result in the overactivation of the PI3K/AKT/mTOR pathway .
A study in human glioma suggested that the loss of PTEN and the consequent upregulation of the PI3K-AKT pathway increased the expression of PD-L1 post-transcriptionally, thus promoting immune resistance . Additionally, other reports validated this resistance in melanoma, prostate and breast cancers, making the inhibition of PI3K-AKT pathway a potential strategy to overcome immunotherapy-resistance .
Chemotherapy drugs kill tumor cells primarily through the induction of apoptosis. The activation of PI3K/AKT/mTOR pathway in tumor cells reduces the pro-apoptotic effect and the cytotoxic effect of chemotherapy drugs, leading to resistance . Therefore, inhibition of this signaling pathway may enhance the sensitivity of chemotherapy drugs.