What Happens When A Mesh Surgery Gets Complicated
Carol, a 77-year-old Ohioan, told me that her transvaginal mesh surgery worked, but only for about 2 years. Ten years ago, when Carol met with her uro-gynecologist for an exam before the surgery, she didnât feel like he had the best bedside manner. Still, she proceeded with the surgery. After having a hysterectomy over 30 years ago and coping with pelvic organ prolapse, Carol said her leaking problems got worse. She was tired of wearing disposables to deal with dribbles and didnât want her prolapse to get worse. âWearing pads got so expensive and frustrating,â she said. âI wanted to improve this problem because nothing else was working.â
But then, two years after surgery, Carol felt pain in her pelvic region. Then, she realized that she was bleeding vaginally. âThe pain was excruciating,â she said. When she saw the mesh coming out, she realized that it was the source of her sudden pain.
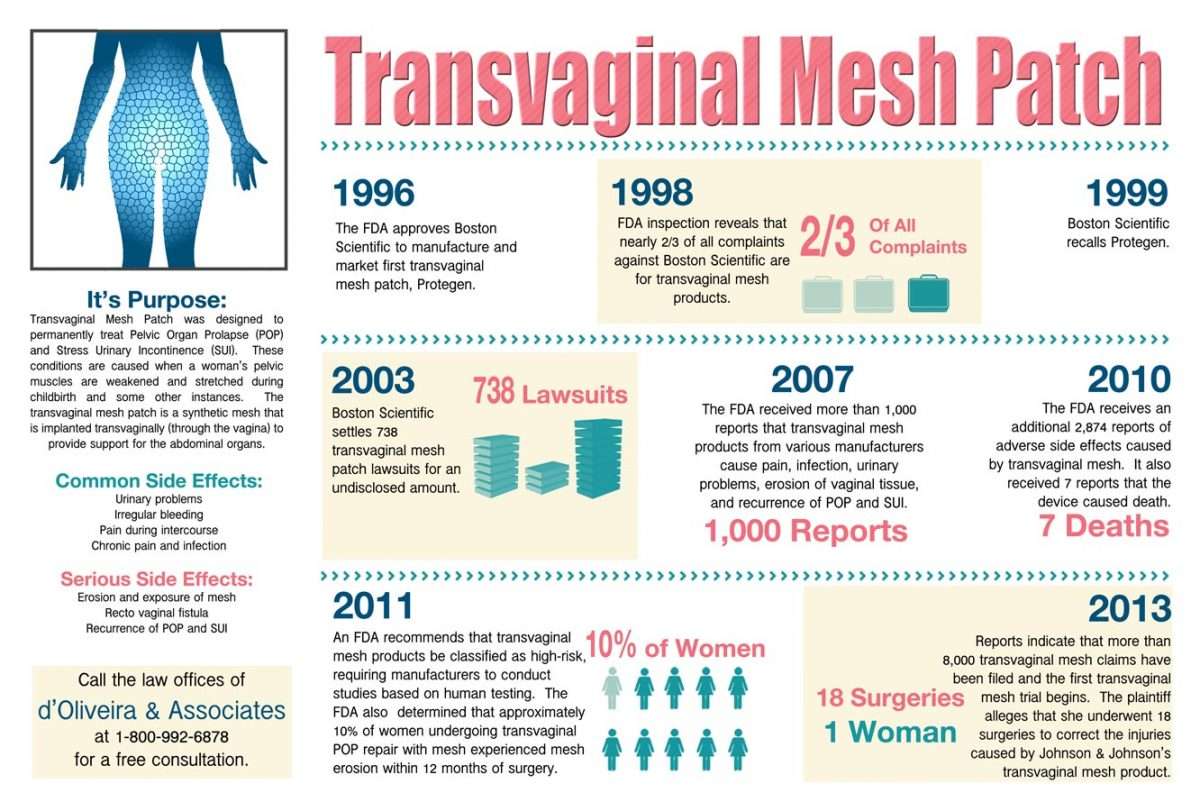
Some women like Carol who have had transvaginal mesh have also experienced pain, bleeding, or erosion . The FDA issued warnings about it in 2008 and 2011. Finally in 2014, they released another notice stating that surgical mesh for transvaginal pelvic organ prolapse should be reclassified from a moderate-risk to high-risk surgery.
Thankfully, Carol had no scarring from her transvaginal mesh surgery, but she still copes with leakage.
Also Check: Natural Remedies For Cystitis Bladder Infection
Vaginal Mesh Has Caused Health Problems In Many Women Even As Some Surgeons Vouch For Its Safety And Efficacy
Regina Stepherson needed surgery for rectocele, a prolapse of the wall between the rectum and the vagina. Her surgeons said that her bladder also needed to be lifted and did so with vaginal mesh, a surgical mesh used to reinforce the bladder.
Following the surgery in 2010, Stepherson, then 48. said she suffered debilitating symptoms for two years. An active woman who rode horses, Stepherson said she had constant pain, trouble walking, fevers off and on, weight loss, nausea and lethargy after the surgery. She spent days sitting on the couch, she said.
In August 2012, Stepherson and her daughter saw an ad relating to vaginal mesh that mentioned 10 symptoms and said that if you had them, to call a lawyer.
My daughter said, Oh mom you have every one of those, Stepherson, of Tyler, Tex., recalled.
Vaginal mesh, used to repair and improve weakened pelvic tissues, is implanted in the vaginal wall. It was initially in 1998 thought to be a safe and easy solution for women suffering from stress urinary incontinence.
But over time, complications were reported, including chronic inflammation, and mesh that shrinks and becomes encased in scar tissue causing pain, infection and protrusion through the vaginal wall.
Chrissy Brajcic, a Canadian who struggled for four years with persistent infections following a mesh implant, became the face of mesh victims with a Facebook page. Brajcic died in December 2017 from sepsis at age 42.
Frequently Asked Questions About Slings
Q: What is stress urinary incontinence and is it a common problem?A: Stress urinary incontinence is loss of urine that occurs at the same time as physical activities that increase abdominal pressure . These activities can increase the pressure within the bladder, which behaves like a balloon filled with liquid. The rise in pressure can push urine out through the urethra, especially when the support to the urethra has been weakened this is what we call stress urinary incontinence. Approximately 1 out of 3 women over the age of 45, and 1 out of every 2 women over 65 have SUI.
Q: What are surgical treatment options for stress urinary incontinence?A: Surgeons have developed different techniques for supporting the bladder back to its normal position. The three main types of surgery are: retropubic suspension and two types of sling procedures.
Retropubic suspension uses surgical threads called sutures to support the bladder neck. In this operation, the surgeon makes an incision in the abdomen a few inches below the navel and then secures the threads to strong ligaments within the pelvis to support the urethral sphincter. This common procedure is often done at the time of an abdominal procedure, such as a hysterectomy.
Q: Has the FDA recalled slings?A: No, the FDA has not recalled slings.
You May Like: How To Take Care Of A Bladder Infection At Home
Also Check: Bladder Leakage Pads For Men
United States Food And Drug Administration Manufacturer And User Facility Device Experience On Use Of Vaginal Mesh In Female Pelvic Floor Reconstruction
MAUDE data represents reports of adverse events involving medical devices. The data consists of all voluntary reports since June 1993, user facility reports since 1991, distributor reports since 1993, and manufacturer reports since August 1996 and is updated on a monthly basis. There are more than 2310 complications reported with the search criteria of vaginal mesh till March 2011. The incidence of complications reported under various search criteria till March 2011 is given in Table 17. A steep increase in the incidence of reported complications with search criteria vaginal mesh and mesh erosion is noted in the MAUDE database .
Recommended Reading: What Causes Overactive Bladder In Women
The First Vaginal Mesh Lawsuits Go To Trial

C.R. Bard withdrew its Avaulta Plus vaginal mesh from the market in July 2012, weeks before losing a $3.6m verdict to a woman who experienced complications from the device.
Johnson & Johnson lost the first federal mesh lawsuit in February 2013, with American Medical Systems becoming the first company to agree to a large settlement in July 2013. Bard lost the second federal lawsuit involving transvaginal mesh less than a month later.
Read Also: What Can You Do For An Overactive Bladder
The Dark History Of Bladder Slings
According to a statement from the FDA, complications from the transvaginal mesh, also known as bladder slings, are not rare. After receiving nearly 4000 patient complication reports and studies that showed the risk involved with bladder slings, the FDA issued a warning for their use in July 2011.
In just a few months from then, early 2012, FDA then sent letters to several manufactures of the bladder slings to require them to have additional tests to see if their products are truly safe for the general public to use and to determine if they posed a high risk of injury.
What Are The Different Types Of Sling Surgery
The two most common types of bladder slings are midurethral and traditional. Midurethral: This sling is inserted during an outpatient procedure that only takes about 30 minutes. Typically, a strip of synthetic mesh is inserted through a single incision in your vagina, and cradles the urethra like a hammock. The procedure may also involve a retropubic method in which two additional incisions are made near the pubic bone to secure the mesh.The transobturator method is another midurethral option in which small incisions are made in the vagina and the labia to secure your sling.Traditional: A traditional, or conventional, bladder sling is inserted during an inpatient procedure, which means you will likely spend a night in the hospital afterward. Traditional bladder sling surgery may use synthetic mesh, or tissue from your own body . With this method, one incision is made in your vagina and one in your belly, in order to connect the sling using tension under the bladder neck. Conventional bladder sling recovery can take longer and may involve more complications.
You May Like: Unable To Hold Urine When Bladder Is Full
What Is A Transvaginal / Pelvic Mesh
Transvaginal Mesh is a small, sturdy fabric-like implant used to repair such issues such as pelvic floor disorders or pelvic organ prolapse . The FDA describes POP as a condition in which internal organs such as the bladder or uterus slip from their usual position and descend into the vagina. This can happen at any age but is most frequently caused by childbirth or genetics. Likewise, pelvic floor disorders have to do with the muscles around the vagina that hold everything in place. Sometimes, these muscles may become stretched or weakened too much to do their job and that is when doctors may have implanted a mesh or a bladder sling to help strengthen them again.
Avaulta Gynecare Other Bladder Slings Targeted By Litigation
Attorneys across the country are handling lawsuits on behalf of patients who were implanted with vaginal mesh products and bladder slings used in the surgical repair of POP or SUI. Many women who underwent vaginal mesh surgery may be unaware of the type of mesh or bladder sling used in their procedure. If you do not know which product you received, your attorney may be able to help you request copies of your medical records to determine the exact product used in your surgery.
The following manufacturers are facing thousands of lawsuits over their vaginal mesh and bladder sling products:
- C.R. Bard, which manufactures:
A few of these mesh products have been recalled or discontinued due to high failure rates, design flaws, or other safety issues.
You May Like: What Happens With Bladder Cancer
Transvaginal Mesh Removal At A Glance
- Women who are suffering from pelvic organ prolapse may opt for surgery to treat serious symptoms that do not improve with lifestyle changes or other devices to help support the prolapsed organs.
- Several surgical methods of repairing pelvic floor disorders exist, including a method known as transvaginal mesh implantation.
- Frequently reported complications from transvaginal mesh include chronic pain, infection, bleeding, pain during intercourse, urinary problems, and exposure of the mesh through the vagina.
- Transvaginal mesh removal is a technically complex surgical procedure in which surgeons attempt to remove as much of the mesh as possible.
- Complete transvaginal mesh removal is possible for some women, while only part of the mesh can be removed in other women due to complicated issues from the type of mesh that was originally used.
What Can I Expect To Receive As Compensation
All legal fee agreements generally have a line that says “Past performance does not indicate future rewards.” This means that even a rainmaker can’t promise you anything. All an attorney can do is provide a range, telling you which clients got A and how many got B. Some cases go to trial and a jury awards damages others settle prior to a verdict. Those settlements have reached $20 million for a single plaintiff, like the one against Ethicon. However, other plaintiffs have had their cases tossed out for lack of evidence. All of this means that nobody can predict anything and those who do are lying.
Recommended Reading: Home Remedies For Kidney And Bladder Infection
Tga Undertakes Regulatory Actions After Review Into Urogynaecological Surgical Mesh Implants
17 May 2019
The TGA decided on 28 November 2017 to remove transvaginal mesh products whose sole use is the treatment of pelvic organ prolapse via transvaginal implantation from the Australian Register of Therapeutic Goods .
This follows a review by the TGA of the latest published international studies and an examination of the clinical evidence for each product included in the ARTG and supplied in Australia. Based on this new information, and since the publication by the TGA of the Results of review into urogynaecological surgical mesh implants, the TGA is of the belief that the benefits of using transvaginal mesh products in the treatment of pelvic organ prolapse do not outweigh the risks these products pose to patients.
As a result, the TGA has taken a series of regulatory actions in relation to transvaginal mesh products and single incision mini-slings . Information about any further actions can be found in the Tables.
The TGA also considers that there is a lack of adequate scientific evidence before the TGA for it to be satisfied that the risks to patients associated with the use of mesh products as single incision mini-slings for the treatment of stress urinary incontinence are outweighed by their benefits. These products will be removed from the ARTG. It should be noted that mini-slings are different devices to mid-urethral slings, which are not being removed from the ARTG.
Table 1. Regulatory action effective 4 January 2018
| ARTG |
|---|
Complications During Or Shortly After Surgery
Intraoperative or perioperative complications occur during surgery or shortly after. In general, these are rarer. Complication rates range from less than 1 percent to 14 percent, according to Costantini and colleagues. Major complications such as vascular and nerve injuries and gut lesions occurred in less than 1 percent of women. Minor bladder injuries had rates from 0.5 to 14 percent. Significant blood loss occurred in about 2.7 percent to 3.3 percent of women.
You May Like: The Most Frequent Initial Symptom Of Bladder Cancer Is
Which Mesh Lawyers Run This Website
Im not a an actor on a television commercial or someone in a call center: Im a real lawyer who has successfully tried serious injury and wrongful death cases in front of juries, and a four-time winner of the American Bar Association Journals award for legal writing. If you use the contact form below, the email goes to me. If you call 948-2718, it will be answered by me or by my receptionist.
But I know the reality: drug companies rarely listen to individual lawyers. I am of counsel with TorHoerman Law, the nationwide product liability firm that negotiated the $2.4 billion Actos settlement and the $650 million Pradaxa settlement. We will bring to your case the same resources, perseverance, and exceptional legal representation.
Vaginal Mesh Complications: Eroded Mesh Infection
Surgical meshes have been used on thousands of women to treat pelvic organ prolapse and stress urinary incontinence . These conditions are a result of the relaxation of tissues that hold the bladder, bowels, and reproductive organs in place, causing them to slide forward and downwards. This can place pressure on the bladder, causing incontinence, and cause vaginal prolapse. Organs may prolapse close to or even outside the vaginal opening. This condition is not only unsightly and distressing it can cause severe discomfort and disrupt sexual function. Transvaginal mesh is often used to reinforce the weakened vaginal walls and give support to the pelvic organs.
Pelvic organ prolapse repair surgery can be performed through the abdomen or the vagina, using stitches or a surgical mesh to reinforce the repair and to support the pelvic organs. According to the FDA, the transvaginal placement of the surgical mesh may put women at a greater risk for POP mesh complications than other surgical options. In addition, the agency claims that with the exposure to greater risk comes no indication of greater clinical benefit in women electing to undergo pelvic organ prolapse repair surgery transvaginally.
The following are among the vaginal mesh complications reported by womenwho underwent pelvic organ prolapse repair surgery with a trans vaginalmesh:
- Mesh erosion
- Contraction or shortening of the vagina
- Discomfort
- Vaginal scarring
- Bowel, bladder and blood vessel perforation
Recommended Reading: Treating Overactive Bladder In The Elderly
Lawsuits Regarding Bladder Slings
So far, over 50,000 cases are known to have been filed against these pelvic mesh products and most of them in the southern district of West Virginia. Under the direction of the US District Judge Joseph L. Goodwin, most were consolidated under eight different multidistrict litigations.
A case list released by the US JPML shows us that Judge Goodwin is currently overseeing a total of 152 cooked medical mesh lawsuits, 6,172 lawsuits against Bard Avaulta, 7,617 lawsuits against Boston scientific 1,155 against Coloplast, 13,000 against Ethicon, and 13,292 against American medical systems.
Judge Goodwin was also assigned to manage the neomedic pelvic mesh lawsuits in February.
Complications That Led To Lawsuits And Settlements
As with any type of surgery, the insertion of a bladder sling comes with risks of complications. Any surgery may lead to bleeding, blood clots, or infections, but some of the complications associated with bladder sling surgery are much more serious and have been the subjects of major lawsuits and the resulting settlements for the plaintiffs.
One of these serious complications is called erosion and occurs when the mesh of the bladder sling erodes or wears through the surrounding tissue inside the body. This can be very painful, but it also may not cause any symptoms until the mesh is so embedded in the tissue, surgery to remove it becomes nearly impossible.
If erosion is not detected and corrected quickly, the mesh can migrate out of place and perforate other tissues and organs. The organs most affected by this are the bladder and urethra, colon, and rectum. The consequences of both erosion and perforation can include severe pain, swelling and infection, bleeding, and damage to tissues and organs that requires surgery, often multiple surgeries. In extreme cases, perforation of the colon or rectum can cause sepsis as bacteria spread through the body.
Don’t Miss: Malignant Neoplasm Of Bladder Unspecified
Bladder Sling Side Effects
A bladder sling is a medical device used to treat certain conditions related to the weakening of tissues and muscles around the bladder. This is mostly a concern for women, and typically in women who have given birth. A bladder sling is a piece of surgical mesh, and although it is not much different from other types of surgical mesh, the way in which it is used can lead to serious complications.
Bladder sling side effects have caused some women serious harm, long-lasting damage, multiple surgeries, and months to years of pain and suffering. In some cases it may be the bladder sling itself, which may be defective, or it may be the procedure used to insert the device that causes such severe complications. Either way, the women affected have filed numerous lawsuits over the side effects caused by various bladder slings.
When Were They Introduced
The most common form of implant, called a transvaginal tape , has been widely used to treat stress incontinence across Europe, the US and Australia since the early 2000s.
Early clinical trials suggested excellent efficacy and many surgeons saw advantages over traditional open-surgery procedures, which took longer to perform, involved a longer recovery for patients and were associated with their own range of complications. By contrast, a TVT procedure typically takes 3o minutes, is performed using keyhole surgery and patients often go home the same day.
Meanwhile, the traditional treatments for pelvic organ prolapse, which included suturing to reconstruct and repair the affected organs and surrounding tissue, were proving less successful, with reports of up to 29% of women suffering another prolapse after treatment. Hysterectomy is another treatment option, which some women wish to avoid.
Because outcomes of using the mesh for incontinence and hernia were so good people were enthusiastic and confident it would also be good for prolapse, said Christopher Maher, a urogynaecologist and associate professor at the University of Queensland. Thats what the mindset was when it was introduced for prolapse around 2002.
But he said regulators around the world should have demanded more testing to ensure the mesh was as effective for treating prolapse as it was for other conditions.
Also Check: What Herbs Are Good For Bladder Control
Don’t Miss: Harmony Urinary Tract And Bladder Support