Natera Accepts All Commercial And Public Insurances
- Benefits include serial use of Signatera in patients with stages II/III colorectal cancer 1
- Benefits also include single time point use for patients with stages II/III colorectal cancer
- Medicare patients with staged II/III CRC are fully covered, other indications are welcome however an ABN may be required*
- Patients on immunotherapy will be covered for serial use of Signatera
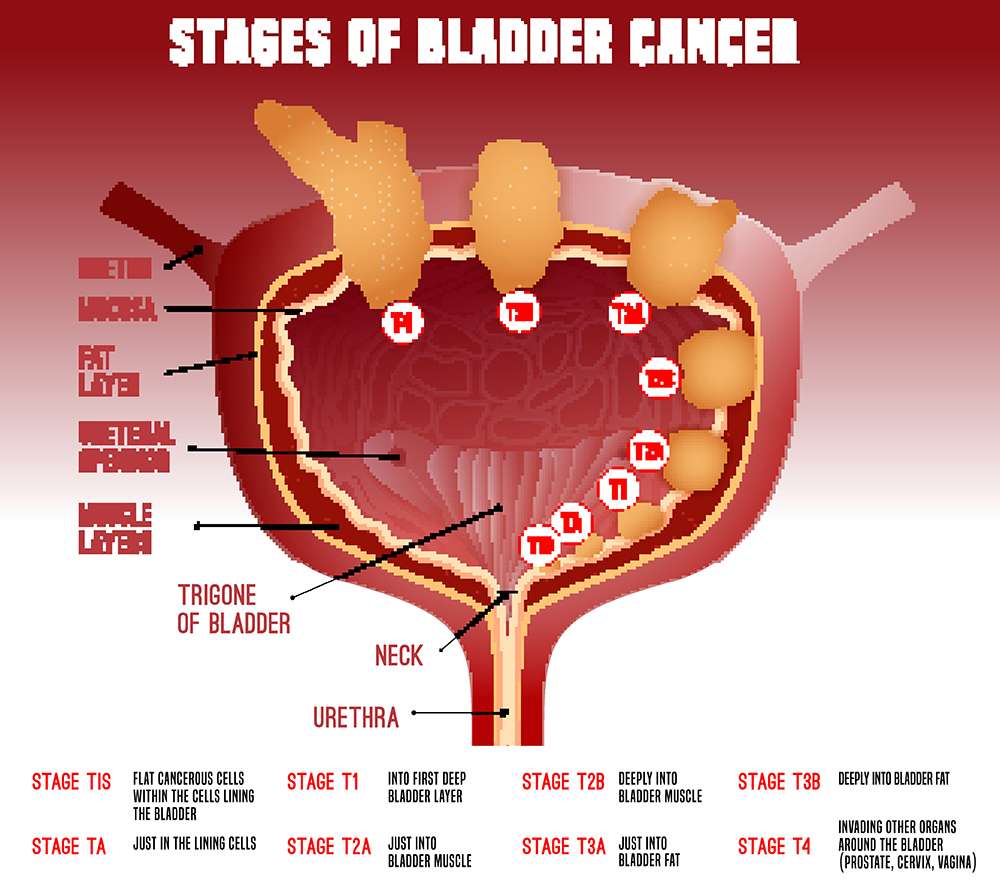
Definition Of Bcg Failure
Recognizing when BCG has failed is another area that can lead to confusion. Although the assumption seems logical that any tumour recurrence after therapy is a ‘BCG failure’, not all patients in this population have a similar prognosis. This situation has immense implications on options for alternative therapy, including the need to proceed to cystectomy. Definitions to clarify what constitutes BCG failure have been published. Herr & Dalbagni and O’Donnell & Boehle suggested definitions of the terms BCG relapse, BCG-refractory and BCG-intolerant:
BCG relapse is considered a recurrence of tumour after a period of disease-free status. Most experts agree that the time point for evaluation should be at 3 months for papillary tumours and 6 months for CIS . Relapse can be further stratified as early , intermediate or late , as the disease-free interval is a prognostic variable early-relapsing patients are more likely to progress and late-relapsing patients can possibly derive some benefit from reinduction with BCG.
BCG-refractory is the persistence of disease after adequate induction and one maintenance course of BCG. Of note, this category includes any progression in stage or grade by 3 months if patients received induction BCG only.
BCG-intolerant is defined as the inability to tolerate at least one full induction course of BCG. The tumour recurs largely because of inadequate therapy, which does not have the same negative prognostic implications as a true BCG failure.
Transurethral Resection Of The Bladder Cancer Tumor
This is when the tumor is removed from the urinary tract through the urethra using an electrical force. Transurethral resection is an endoscopic or scope procedure that does not involve making an incision in the body.
Drug therapy after TUR is commonly prescribed for patients with large, multiple or high-grade tumors.
Recommended Reading: How To Detect Bladder Cancer Early
Part Ii Drug Disposition During Intravesical Therapy
Drug disposition in the bladder during intravesical therapy is affected by several attributes, i.e., physicochemical properties of the drug , urine volume and pH, patient hydration status, and integrity of urothelium. Our laboratory has provided the first pharmacokinetic models to describe drug disposition in urine and bladder tissues. These models enable the prediction of changes in drug concentration in different parts of bladder wall as a function of physiological, pathological or pharmacological parameters .
The first set of equations describes the urine pharmacokinetics during treatment:
Submucosa/superficial muscle layer :
The above urine and tissue pharmacokinetic models provide the basis for computing drug delivery to the targeted, tumor-residing sites in the bladder as a function of treatment conditions , during intravesical therapy. Integration of drug delivery with pharmacodynamic data such as the effective drug concentrations in preclinical models provides a means to rational design of intravesical treatments .
Purification And Refolding Of Sa
SA-GM-CSF fusion protein was collected as inclusion body after cell lysis by sonication, and washed consecutively for 5 min. on a rotating platform with each solution: A , B , C , D and E . After washing, the inclusion body was dissolved in 8M urea buffer , and purified on the Ni-NTA column according to the manufactures protocol. The purified SA-GM-CSF fusion protein was adjusted to 0.2 at OD280, dialysed against the refolding buffer . The bound SA-GM-CSF fusion protein was eluted with 50 mM NaAc, 150 mM NaCl, pH 4.0, adjusted to pH 8.0 with 500 mM 2CO3, 5.0 M NaCl, pH 11, and then passed through Detoxi-Gel Endotoxin Removing Gel to remove bacterial endotoxin contaminants. The amount of the SA-GM-CSF fusion protein in the preparations was quantitatively measured by use of mouse GM-CSF Single Analyte ELISArray Kit .
Similarly, SA-tagged green fluorescence protein named SA-GFP fusion protein was expressed, purified and refolded.
Recommended Reading: How Can I Treat A Bladder Infection At Home
Predictors Of Response To Bcg
Markers of response to intravesical BCG fall into three broad categories: determination of response , use of surrogate endpoint biomarkers and prediction of response. BCG is most frequently used to treat high-grade disease and assessing the response of CIS specifically is challenging because it is often difficult to detect. A commonly used design is to determine response by cystoscopy, cytology and possibly bladder biopsy at 3 months and 6 months following a BCG induction regimen. However, the 3-month assessment is problematic because of the well documented conversion of positive cytology at 3 months to negative cytology at 6 months, particularly after maintenance BCG. Fluorescence cystoscopy can improve detection of both CIS and papillary bladder cancer, but fluorescence can also be induced by inflammation. It remains to be seen whether fluorescence cystoscopy can reliably improve the determination of BCG response.
Cytology is a subjective test with variable performance molecular tests that are more objective might yield better and more consistent performance in detecting visually occult bladder cancer. Patients who have abnormal results in the UroVysion assay in a urine sample obtained immediately before the last of the 6-week induction instillations have increased risk for tumour recurrence and progression.
Bcg Strains: Is There A Difference
There have been several strains of BCG developed since the original strain in 1921, but whether these strains have varying efficacies on bladder tumours remain unclear. While hundreds of thousands of patients have been treated with BCG for prevention of NMIBC, no clinical difference has been shown among studies despite the use of various strains worldwide.
Trials using various strains have consistently demonstrated the efficacy of BCG immunotherapy in reducing recurrence and progression of NMBIC in all countries across the globe. BCG is recommended by all scientific associations from the European Association of Urology , American Urological Association, Japanese Association or the Canadian Urological Association.
Sengiku and colleagues demonstrated no significant difference between the studied strains . Supporting a previous European Organization for Research and Treatment of Cancer meta-analysis which suggested that there is no large difference between different strains , a recent meta-analysis of randomised trials performed by Quan et al concluded that no meaningful correlations between BCG strain and other survival outcomes could be drawn. Another systematic review and meta-analysis by Chou et al concluded that no comment can be made regarding differences among strains. Gan et al studied the effects of substrain differences in BCG immunotherapy for bladder cancer and came to a similar conclusion.
You May Like: Can A Bladder Infection Make You Nauseous
Intravesical Therapy For Bladder Cancer
With intravesical therapy, the doctor puts a liquid drug right into your bladder rather than giving it by mouth or injecting it into your blood. The drug is put in through a soft catheter that’s put into your bladder through your urethra. The drug stays in your bladder for up to 2 hours. This way, the drug can affect the cells lining the inside of your bladder without having major effects on other parts of your body.
What Is Bcg Treatment
Bacillus Calmette-Guerin treatment is a type of intravesical immunotherapy. This liquid drug is made from a strain of Mycobacterium bovis the same bacterium used to create the tuberculosis vaccine. When used in medicine, Mycobacterium bovis is weakened to reduce harm to your body.
BCG treatment is usually given after TURBT , which is a bladder surgery to remove any visible cancer.
You May Like: How To Fix Bladder Leakage After Pregnancy
Review Of Current Guidelines On Bcg Use
Several guidelines exist regarding the use of BCG in NMIBC, such as those by the American Urological Association , the European Association of Urology , the National Comprehensive Cancer Network , the International Bladder Cancer Group and the International Consultation on Urological Diseases . Even though each of these guidelines is based on large-scale literature reviews on similar available data, the guidelines are somewhat disparate, making implementation of the recommendations into routine practice difficult for the practicing urologist.
Intravesical Immobilization Of Sa
The mouse orthotopic model of MB49 bladder cancer was used to evaluate the feasibility and efficacy of the novel immunotherapy through intravesical immobilization of SA-GM-CSF bifunctional fusion protein on the biotinylated mucosal surface of bladder wall. Briefly, 8- to 10-week-old female C57BL/6 mice, weighing 1520 grams, were anaesthetized with intraperitoneal sodium pentobarbital . Subsequently, a 24-gauge Insyte IV catheter was inserted through the urethra into the bladder using mineral oil as a lubricant. In order to prepare the bladder for tumour implantation, the pretreatment was performed, i.e. a brief acid exposure , followed by alkaline neutralization , which caused chemical lesions on the bladder mucosal surface. The content was washed out by transurethral infusion of 1 × PBS. After the pretreatment, 100 l of MB49 cells were instilled and allowed to incubate in the bladder for 1 hr. One day after tumour implantation, 100 l of 1 mg/ml NHS-PEO4-biotin was instilled and allowed to incubate in the bladder for 30 min., followed by intravesical instillation of 100 l of 1 × PBS, GM-CSF , SA-GFP , or SA-GM-CSF and incubation for 1 hr. The SA-GM-CSF group had 16 mice and other groups had 10 mice individually. The treatment was performed twice a week for 3 weeks. Mice were carefully monitored for health status , haematuria and palpable tumour.
Read Also: Galvanized Pressure Tank Vs Bladder Tank
Radiation As An Option For Recurrent Disease
Cystectomy remains the standard for recurrent BCG-refractory, high-grade T1 bladder tumours. However, many patients are not undergoing surgery, possibly owing to comorbidities and/or a desire to avoid a large operation. Furthermore, the high rates of clinicopathological stage discordance noted after surgery might contribute to the poor long-term results of second-line intravesical agents following BCG failure.
Radiation therapy presents a non-surgical option for BCG failures. Early studies showed reasonable response rates ,, and one randomized trial in patients with high-grade T1 tumours comparing conservative treatment with radiation alone showed no difference between treatments in terms of recurrence.
Based on current evidence, the new 2015 NCCN bladder cancer guideline states that external-beam radiotherapy is rarely appropriate for patients with stage Ta, T1, or Tis disease. Radiochemotherapy might be a potentially curative alternative to cystectomy in patients with recurrent TaT1 disease . Currently, the North American multicentre, cooperative Radiation Therapy Oncology Group protocol RTOG 0926 is evaluating the role of radiochemotherapy after maximum TURBT for patients with high-risk T1 bladder cancer following BCG failure for whom the next therapy would have been radical cystectomy.
Drugs To Affect Absorption
Several drugs have been proposed to enhance the permeability of the bladder wall in order to increase the local drug concentration. These include dimethyl sulfoxide , Tween 80 and hyal-uronidase.
DMSO is a dipolar solvent that is instilled into the bladder for interstitial cystitis. An in vivo study indicated that pretreatment with DMSO increased the absorption of chemotherapy instilled afterwards . There is also evidence that DMSO can increase the anticancer activity of
Don’t Miss: How Are Bladder Infections Caused
Bcg Failure And Subclassification
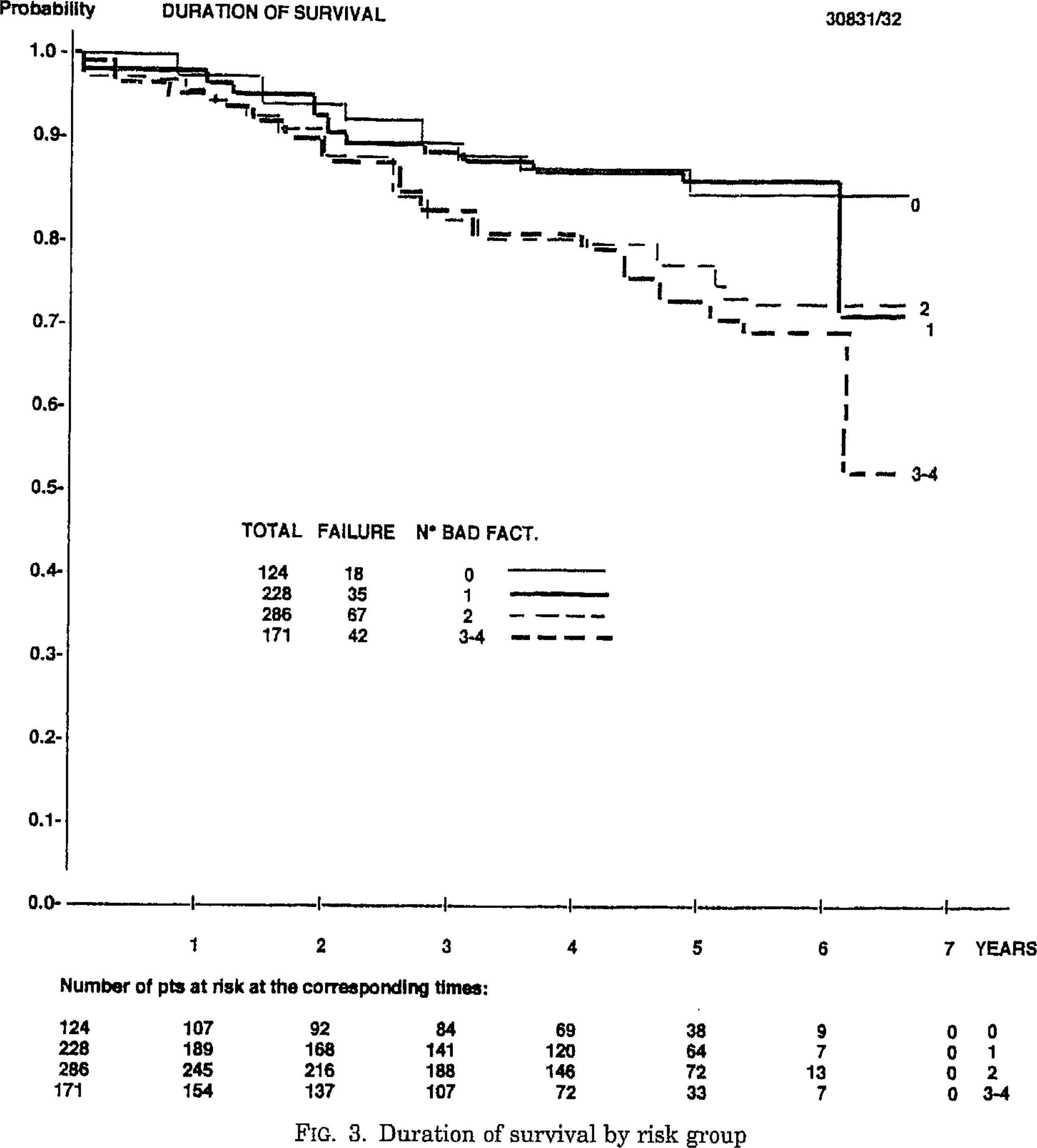
About 40% of patients of NMIBC will fail intravesical BCG treatment. Although many factors might lead to BCG failure, the dose of BCG and type of T helper response may lead to dramatically diverging outcomes. Low-dose BCG might not trigger enough TH1-type immune response, which is the main response to BCG activity. Too high doses of BCG may paradoxically activate mixed TH1/TH2 responses which will counterbalance the TH1 response . Other factors of BCG failure include occult micrometastatic disease prior to BCG therapy .
Patients who fail intravesical BCG treatment are usually sub-classified into three categories based on the type of failure:
BCG refractory, which is the persistence of disease after induction or maintenance BCG treatment.
BCG relapse, the recurrence of disease after a disease-free period post BCG treatment.
BCG intolerance when the patient is not tolerating the completion of BCG induction .
The definitions, endpoints and clinical trial designs for NMIBC as recommended by the International Bladder Cancer Group might serve as an excellent current state-of-the-art resource . The type of failure should be clearly defined. Because stakes are very high for these patients, for whom BCG has failed, and options are limited, single-arm designs may be relevant for the BCG-unresponsive population. The consensus for a clinically meaningful initial complete response rate or recurrence-free rate is of at least 50% at 6 months, 30% at 12 months and 25% at 18 months.
Practical Issues Of Bcg Administration
Some practical points need to be considered when using BCG for bladder cancer treatment. BCG manufacturers recommend evaluating the tuberculosis status of the patient with a PPD tuberculosis skin test before initiation of therapy, with some practitioners obtaining chest radiographs in all patients. This procedure has never proven necessary, probably owing to the exceedingly low incidence of tuberculosis in developed nations, where most bladder cancer patients do not meet the criteria for disease screening as recommended by the Centers of Disease Control and Prevention. In fact, patients with a positive PPD test without active disease should not be excluded from BCG therapy, as the presence of a systemic immune response might help augment the antitumour response as noted above. In addition, patients with a positive PPD result were shown to display adverse effect profiles during BCG treatment that are similar to patients with a negative test.
Recommended Reading: Treatment For Overactive Bladder In Males
What Does Residual Disease Detection Offer4
After a recurrence of bladder cancer, Bonnie and her doctor used Signatera to help monitor how well her immunotherapy treatment was working. Signatera testing helped guide important treatment change decisions and provided comfort to Bonnie.
Getting the results of made me feel comfortable in enjoying life. Bonnie Miller
Is There A Risk Of Bladder Cancer After Bcg Treatment
Like most cancers, bladder cancer can potentially return after treatment. Statistically, cancer will recur in up to 40% of people who receive BCG treatment. Its important to note, however, that even if the cancer comes back, it may not progress.
Low-grade bladder cancer usually doesnt spread to other areas of your body. But people who have low-grade bladder cancer have a higher risk of developing other low-grade cancers throughout their lifetime.
Less often, aggressive bladder cancer can develop after BCG treatment. If this happens, cystectomy is usually recommended.
Read Also: Whats The Difference Between A Uti And A Bladder Infection
Mechanism Of Action Of Bcg
The mechanism of action of bacillus Calmette-Guérin therapy is incompletely understood. Some early studies purported that an immune response against BCG surface antigens cross-reacted with putative bladder tumor antigens, and this was proposed as the mechanism for the therapeutic effect of BCG however, multiple subsequent studies refute this claim.
The most likely mechanism of action of BCG immunotherapy involves a combination of its direct effect on tumor cells along with the patients immune response to the therapy. These effects are summarized by Kawai et al into three categories: infection of cancer cells, induction of immune response, and antitumor effects.
The infection of cancer cells is mediated by the glycoprotein fibronectin, which allows the internalization of BCG, breakdown of proteins, and cellular changes that trigger the immune system. This is similar to the immunologic reaction that occurs in patients with tuberculosis. This immune response comprises specific cellular changes including surface receptor changes and release of various cytokines. Interferon is considered to be an important part of this process and has been used in the past to determine appropriate response to treatment. The immune response crescendos to antitumor activity in which cells recognize the cancer cells, target them for destruction, and subsequently decrease cancer burden.
The overall response to BCG is limited if the patient is immunosuppressed.
Intravesical Bcg Dose And Schedule
To obtain the standard dose, the BCG vaccine powdered vial is usually diluted into 50 ml of normal saline. The diluted BCG is then infused into the bladder through a urethral catheter after complete drainage of the bladder. It should be maintained in the bladder for 2 hours. BCG is administered for 2 to 4 weeks after resection to prevent the risk of systemic toxicity . The schedule of intravesical BCG treatment comprises an induction course and a maintenance course .
We have previously shown that in most patients, the maximal peripheral immune response is already observed after 4 weekly BCG instillations. However, patients not previously immunised against mycobacterial antigens may require 6 weekly instillations to achieve a maximum stimulation level . Following the induction course, several studies have reported that additional BCG treatment may decrease recurrence.
Two decades ago, Zlotta et al showed that intravesical BCG instillations induced a transient peripheral immune activation against BCG antigens. Reactivation was observed in most cases after additional BCG courses . This absence of long-lasting immune activation after a single 6-week course of BCG could be related to the increased clinical efficacy observed with BCG maintenance instillations. However, the optimum period of BCG maintenance is still controversial. The Southwest Oncology Group BCG maintenance regimen was a weekly dose for 3 weeks at 3, 6, 12, 18, 24, 30 and 36 months .
Also Check: How Is Botox Administered For Overactive Bladder
Part Iv Experimental Approaches
Various experimental approaches in two general categories have been evaluated. Approaches in the first category share a common theme of improving the total drug exposure, by enhancing the delivery of agents to bladder tissues , prolonging the exposure , or enhancing cell membrane permeability . The second category is gene therapy, with the goal of either correcting the mutated and malfunctioned genes responsible for tumor formation and progression or as a means to deliver intrinsic or extrinsic signals for cell destruction.
Intravesical Therapy Of Superficial Bladder Cancer
Volume 6, Issue 3, 2000
Page: Pages: 15
Abstract
Title: Intravesical Therapy of Superficial Bladder Cancer
Volume: 6Issue: 3
Michael D. Melekos and George D. Moutzouris
Affiliation:
- Agios Georgios Rio, 60 IroonPolytechniou Str., GR-265 00 Rio , Greece,Greece
Keywords:bladder cancer, transurethral resection TUR, Transitional Cell carcinoma TCC, bacillus calmette guerin BCG, Carcinoma in situ, Intravesical therapy, dysplasia, intravesical chemotherapy, thiotepa, triethylenethiophosphoramine, ethoglucid Epondyl, mitomycin C MMC, Doxorubicin Adriamycin, epirubicin, mitoxantrone, Intravesical Immunotherapy, anthracenedione, interferons INFs, Interleukin 2 IL2, natural killer NK, lymphokine activated killer cell LAK, keyhole limpet hemocyanin KLH, intravesical chemoprophylaxis, BCG Immunoprophylaxis, complete response, delayed type hypersensitivity, electromotive drug administration, isoniazid
Don’t Miss: What Can I Do For A Prolapsed Bladder