Cell Lines Proliferation Assay
The BC cell lines including T24 and 5637 cells were obtained from the American Type Culture Collection . The authentication was obtained by Human STR Profiling Cell Authentication Service, and the mycoplasma test was tested in all cell lines. These cells were maintained in 10% fetal bovine serum supplemented with 2mM of L-glutamine at 37°C in 5% CO2. Cells treated in individual experiments were assessed for cell viability using Cell Titer-Fluor Assay and Caspase-Glo 3/7 Assay following the manufacturers protocol.
Fda Converts Indication For Pembrolizumab As Frontline Therapy For Bladder Cancer To Full Approval
The FDA has granted a full approval to pembrolizumab for the treatment of platinum-ineligible patients with urothelial cancer.
An accelerated approval for pembrolizumab as therapy for patients with locally advanced or metastatic urothelial cancer who are not eligible to receive platinum-based therapies has been converted to full approval following meetings with the FDAs Oncologic Drugs Advisory Committee , reported the drugs developer, Merck.1
As part of an industry-wide effort by the FDA to reassess the status of systemic therapy agents granted accelerated approval and whose confirmatory trials did not denote a clinical benefit to treatment, this indication for pembrolizumab was reviewed with the ODAC, who voted 5 to 3 in favor of maintaining the accelerated approval despite disappointing findings in a phase 3 trial.
While the treatment landscape has evolved, an unmet need remains for appropriate patients newly diagnosed with certain types of advanced urothelial carcinoma who are not eligible for platinum-containing chemotherapy, Scot Ebbinghaus, MD, vice president for clinical research at Merck Research Laboratories, said in a press release. We are confident in the role Keytruda will continue to play for these patients who have few other treatment options and are working with urgency to advance studies to help more patients living with bladder and other types of cancer.
Trial Results Support Ongoing Investigation Of Enfortumab Vedotin
Results demonstrated a 64.5% confirmed objective response rate in patients treated with the investigational combination.
New data from the phase 1b/2 EV-103 clinical trial cohort K support ongoing research into the use of enfortumab vedotin-ejfv in combination with pembrolizumab, and enfortumab vedotin-ejfv alone in patients with unresectable locally advanced or metastatic urothelial cancer who are ineligible for cisplatin-based chemotherapy.
Results demonstrated a 64.5% confirmed objective response rate in patients treated with the investigational combination. The data were highlighted in a late-breaking presentation at the European Society for Medical Oncology Congress 2022.
Nearly 65% of patients who were treated with enfortumab vedotin and pembrolizumab responded to this combination, with almost 11% showing no detectable cancer following treatment, said Margorie Green, senior vice president and head of late-stage development at Seagen, in a press release. These study results represent an encouraging finding for people with advanced urothelial cancer who are not eligible for cisplatin treatment.
The confirmed ORR was the primary endpoint of cohort K, with 10.5% of patients experiencing a complete response and 53.9% of patients experiencing a partial response. The median duration of response was not reached.
REFERENCE
Related Content:
You May Like: What Is The Best Treatment For Bladder Cancer
Biospecimen Collection And Pd
Biospecimens from patients diagnosed with UC by the tumor resection with either transurethral resection or radical cystectomy performed at Osaka Medical and Pharmaceutical University hospital were collected in RNAlater reagent . All the H& E-stained cases were reviewed by a board-certified pathologist to confirm that the tumor specimen was histologically consistent with UC. Tumor sections were required to contain an average of 60% tumor cell nuclei equal to or less than 20% necrosis for inclusion in the study. RNA and DNA were extracted from tumor-adjacent normal tissue specimens using the DNA/RNA AllPrep kit . Quantification of nucleic acids was performed using NanoDrop Microvolume UV-Vis Spectrophotometer . RNA was analyzed via the Agilent 2100 Bioanalyzer for the determination of an RNA Integrity Number , and only the cases with RIN > 7.0 were included in this study.
PD-L1 protein expression in immunohistochemistry was evaluated in obtained tumor samples from the independent cohort using the PD-L1 IHC 22C3 pharmDx assay and the 22C3 ant-PD-L1 antibody . In short, the PD-L1 protein expression is determined by Combined Positive Score , which is the number of PD-L1 staining cells divided by the total number of viable tumor cells, multiplied by 100. The CPS was evaluated by a board-certified pathologist.
Localized/early Transitional Cell Carcinomas Of Bladder
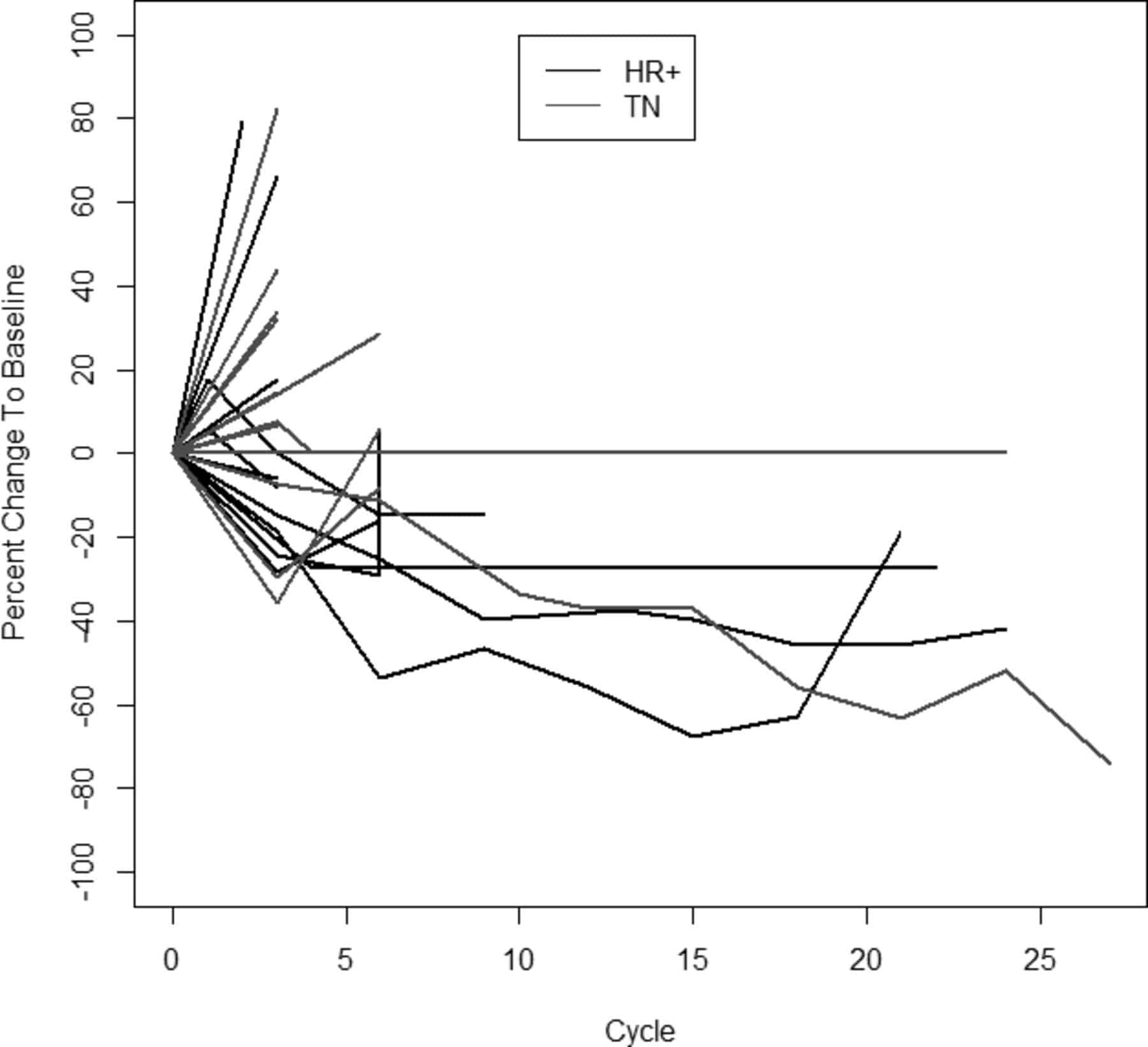
Transitional cell carcinomas can be very difficult to treat. Treatment for localized stage transitional cell carcinomas is surgical resection of the tumor, but recurrence is common. Some patients are given mitomycin into the bladder either as a one-off dose in the immediate post-operative period or a few weeks after the surgery as a six dose regimen.
Localized/early transitional cell carcinomas can also be treated with infusions of Bacille CalmetteâGuérin into the bladder. These are given weekly for either 6 weeks or 3 weeks . Side effects include a small chance of developing systemic tuberculosis or the patient becoming sensitized to BCG, causing severe intolerance and a possible reduction in bladder volume due to scarring.
In patients with evidence of early muscular invasion, radical curative surgery in the form of a cysto-prostatectomy usually with lymph node sampling can also be performed. In such patients, a bowel loop is often used to create either a “neo-bladder” or an “ileal conduit” which act as a place for the storage of urine before it is evacuated from the body either via the urethra or a urostomy respectively.
Recommended Reading: Bladder Cancer Stages And Grades
Keytruda Receives Full Approval As First
The approval follows the FDAs Oncologic Drugs Advisory Committee voting 5-3 in favor of maintaining the approval despite a confirmatory trial that found Keytruda did not meet the end points of overall survival and progression-free survival.
The FDA has provided full approval for Keytruda for first-line advanced urothelial carcinoma. Previously, Keytruda was given accelerated approval for the treatment of locally advanced or metastatic urothelial carcinoma who were not eligible for cisplatin-containing chemotherapy or for patients who were not eligible for any platinum-containing chemotherapy regardless of PD-L1 status
The indication on the label has been revised to be for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for any platinum-containing chemotherapy.
In April 2021, the FDAs Oncologic Drugs Advisory Committee voted 5-3 in favor of maintaining the indication in urothelial cancer despite the fact that the confirmatory phase 3 trial did not meet the dual primary end points of overall survival or progression-free survival. Panelists who voted for keeping the indication suggested that because Keytruda is active as a second-line treatment for patients with platinum-refractory urothelial carcinoma, it could be argued that it is active in the second-line setting.
Fda Grants Full Approval To Pembrolizumab In Frontline Bladder Cancer
The FDA has converted the accelerated approval of frontline pembrolizumab in advanced bladder cancer to a full approval and revised the indication to cover the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for any platinum-containing chemotherapy.1
In May 2017, the FDA initially approved the pembrolizumab for the first-line treatment of patients with locally advanced or mUC who are ineligible for cisplatin-containing chemotherapy.
In 2018, the FDA updated the label for frontline pembrolizumab in this setting, with the approval now being specifically for the treatment of patients with locally advanced or mUC who are not eligible for cisplatin-containing therapy and whose tumors express PD-L1 , or the treatment of patients who are not eligible for any platinum-containing chemotherapy, regardless of PD-L1 status.
The label update today was based on analyses from the phase 3 KEYNOTE-361 trial, which was intended to be the confirmatory trial for the frontline accelerated approval of pembrolizumab in bladder cancer. The trial randomized patients with advanced or mUC to frontline treatment with single-agent pembrolizumab, pembrolizumab plus chemotherapy, or chemotherapy alone.
References
1. FDA Approves Updated Indication for Mercks KEYTRUDA® for Treatment of Certain Patients With Urothelial Carcinoma . Published online August 31, 2021. Accessed August 31, 2021. https://bwnews.pr/3kF1dw6.
Related Content:
Recommended Reading: Robotic Surgery For Prolapsed Bladder
Pembrolizumab Use In Bladder Cancer: A Tale Of Two Trials
volume 18, pages 577578
The treatment of patients with the PD1 inhibitor pembrolizumab yields benefit in the second-line metastatic urothelial cancer setting. Two new trials have studied pembrolizumab monotherapy in other patients with urothelial carcinoma: an open-label phase II trial has enrolled patients with high-risk BCG-refractory non-muscle-invasive disease, and a phase III trial has compared three first-line treatment options for patients with metastatic disease. However, these trials have shown conflicting results.
Refers to Powles, T. et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma : a randomised, open-label, phase 3 trial. Lancet Oncol.22, 931945 | Balar, A. V. et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG : an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol.22, 919930 .
This is a preview of subscription content, access via your institution
Immunotherapys Roles In Treating Metastatic Urothelial Carcinoma
Transcript:
Ignacio Durán, MD, PhD: Theres no doubt that immunotherapy has changed the landscape of bladder cancer in the last 7 to 10 years. It started with the first study with atezolizumab in patients who had been heavily pretreated. In that context we observed some responses in which the drug was stimulating their immune system. That family of drugs, called checkpoint inhibitors, started its development in bladder cancer. We came to the conclusion that a number of compounds were very active in advanced bladder cancer. Two of them, atezolizumab and pembrolizumab , made it to the top of that list because of 2 randomized phase 3 trials. Pembrolizumab was the 1 with the highest level of evidence, and it became the standard of care in patients who have progressed after receiving chemotherapy. That was the first hit of immunotherapy in bladder cancer, becoming the preferred second line.
Transcript edited for clarity.
You May Like: How Do You Get Rid Of Overactive Bladder
Advances And Controversies With Checkpoint Inhibitors In Bladder Cancer
- Encyclopedia of Cancer and Society2007
- The SAGE Handbook of Healthcare2008
- The SAGE Encyclopedia of Cancer and Society2015
- The SAGE Handbook of Healthcare2008
- The SAGE Encyclopedia of Cancer and Society2015
- Encyclopedia of Medical Decision Making2009
- The SAGE Handbook of Healthcare2008
- Encyclopedia of Cancer and Society2007
- The SAGE Handbook of Healthcare2008
Checkpoint Inhibitors And Bladder Cancer
Long-term survival for people diagnosed with advanced bladder cancer is poor, with approximately 5% of patients with metastatic bladder cancer surviving for 5 years or more.
Checkpoint inhibitors have shown activity in patients with metastatic bladder cancer in both the second-line setting and the first-line setting , explained Andrea B. Apolo, M.D., who heads the Bladder Cancer Section in NCIs Center for Cancer Researchs Genitourinary Malignancies Branch.
But we still needand are awaitingthe results of ongoing randomized trials comparing checkpoint inhibitors and chemotherapy in the first-line setting for patients with metastatic bladder cancer, she continued. The results will allow us to adequately compare patient outcome in terms of survival and quality of life with these therapies.
With the exception of pembrolizumab, the drugs covered by these approvals target a protein known as PD-L1 that is expressed at high levels on some cancer cells. Pembrolizumab targets PD-1, the receptor protein for PD-L1, on immune cells. Normally, binding of PD-L1 to PD-1 tamps down immune activity. By preventing the interaction between PD-L1 and PD-1, all four drugs can allow the immune system to be more active against tumor cells.
The other checkpoint inhibitor approved by the FDA for the treatment of patients with bladder cancer, nivolumab , targets PD-1.
Also Check: How Do Doctors Test For Bladder Infection
For Those Who Are Candidates Data Are Lacking On How To Choose Between Available Options
byLeah Lawrence, Contributing Writer, MedPage Today November 22, 2021
There are two immunotherapy options approved for first-line treatment of metastatic urothelial cancer in patients who are platinum-ineligible. In 2017, the FDA granted accelerated approvals to atezolizumab and pembrolizumab for patients with advanced disease who are unable to receive cisplatin chemotherapy — the recommended first-line option.
“Currently, the role of immunotherapy is waning a bit in the frontline space,” said Shilpa Gupta, MD, director of Genitourinary Medical Oncology at Taussig Cancer Institute and co-leader of the Genitourinary Oncology Program at the Cleveland Clinic in Ohio.
Initially, pembrolizumab was approved for metastatic disease that was not eligible for cisplatin-containing chemotherapy. However, FDA limited the labels to patients who were ineligible for cisplatin-containing regimens and had tumors that are PD-L1 high, or patients who were not eligible for any platinum-containing therapy regardless of PD-L1 status, after data from two clinical trials showed worse outcomes with pembrolizumab or atezolizumab compared with platinum-based chemotherapy in patients with low or no PD-L1 expression. The FDA also required the use of FDA-approved companion diagnostic tests to determine PD-L1 levels.
“For atezolizumab, the old label is still in place, but the data are under review by the FDA,” Gupta told MedPage Today.
Ineligibility
- ECOG performance status 3 or higher
Keytruda Gets Full Approval For First
The Food and Drug Administration has granted full approval and revised the indication for Keytruda® for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for any platinum-containing chemotherapy.
Previously, Keytruda was indicated for the treatment of patients with locally advanced or mUC who were not eligible for cisplatin-containing chemotherapy and whose tumors expressed PD-L1 , as determined by an FDA-approved test, or in patients who were not eligible for any platinum-containing chemotherapy regardless of PD-L1 status. This indication was granted accelerated approval based on tumor response rate and duration of response.
The full approval was based on data from the confirmatory phase 3 KEYNOTE-361 trial , which assessed the efficacy and safety of pembrolizumab as monotherapy and in combination with chemotherapy for the first-line treatment of 1010 patients with locally advanced or mUC. Patients were randomly assigned 1:1:1 to receive pembrolizumab plus chemotherapy , pembrolizumab alone, or chemotherapy alone.
Results showed that the trial did not meet the prespecified dual primary endpoints of overall survival or progression-free survival. The combination of pembrolizumab and chemotherapy did demonstrate the following numerical improvements in both primary endpoints after a median follow-up of 31.7 months compared with chemotherapy alone, respectively:
References
This article originally appeared on MPR
Don’t Miss: Things To Avoid When You Have A Bladder Infection
Advanced Or Metastatic Transitional Cell Carcinomas
First-line chemotherapy regimens for advanced or metastatic transitional cell carcinomas consists of gemcitabine and cisplatin) or a combination of methotrexate, vinblastine, adriamycin, and cisplatin.
Taxanes or vinflunine have been used as second-line therapy .
Immunotherapy such as pembrolizumab is often used as second-line therapy for metastatic urothelial carcinoma that has progressed despite treatment with GC or MVAC.
In May 2016, the FDA granted accelerated approval to atezolizumab for locally advanced or metastatic urothelial carcinoma treatment after failure of cisplatin-based chemotherapy. The confirmatory trial failed to achieve its primary endpoint of overall survival.
In April 2021, the FDA granted accelerated approval to sacituzumab govitecan for people with locally advanced or metastatic urothelial cancer who previously received a platinum-containing chemotherapy and either a programmed death receptor-1 or a programmed death-ligand 1 inhibitor.
Onclive: Could You Provide An Overview Of The Results You Presented Here At Esmo
In an interview with OncLive, Balar, director of the Genitourinary Medical Oncology Program at NYU Langone Medical Center, discussed the significant findings from this interim analysis, as well as what that next steps may include.Balar: KEYNOTE-052 was a first-line study of pembrolizumab in cisplatin-ineligible patients with advanced urothelial cancer. Overall, the study enrolled 374 patients. However, what I presented today was the interim analysis of the first 100 patients. The key objectives of this planned interim analysis were to get a sense of what the objective response rate was in the first 100 patients, but also to develop the biomarkerthe PD-L1 expression scorethat would subsequently need to be validated in the remaining patients in the study.
What we found was that the objective response rate in the first 100 patients was 24%, including 6% of patients achieving a complete response. Additionally, when we looked at PD-L1 expression, what we did in this particular trial was look at something called the combined positive score . That score is calculated by looking at both the expression of PD-L1 on tumor cells and infiltrating immune cells, and take that over the total number of tumor cells in the tumor, and reflect that as a percentage.
Were there any noteworthy toxicities associated with pembrolizumab in this study?
What are the next steps following this interim analysis?
Are there any other ongoing studies in this space that youre excited to see the results of?
Also Check: Will Cranberry Pills Help An Overactive Bladder
Fda Approves Pembrolizumab For First
Pembrolizumab has been granted full approval as a first-line treatment for a select population of patients with bladder cancer.
The FDA has granted full approval to pembrolizumab for the treatment of patients with locally advanced or metastatic urothelial carcinoma and are not deemed eligible for platinum-containing chemotherapy.
Pembrolizumabs original indication had been granted an accelerated indication based off the tumor response rate and duration of response with continued approval remaining contingent upon certification of clinical benefit in confirmatory trials.
While the treatment landscape has evolved, an unmet need remains for appropriate patients newly diagnosed with certain types of advanced urothelial carcinoma who are not eligible for platinum-containing chemotherapy, Scot Ebbinghaus, MD, vice president, clinical research, Merck Research Laboratories, stated in a press release. We are confident in the role will continue to play for these patients who have few other treatment options and are working with urgency to advance studies to help more patients living with bladder and other types of cancer.
Earlier this year, the drug was assessed by the FDAs Oncologic Drugs Advisory Committee during an industry wide evaluation of accelerated approvals that had not yet met their post-marking requirements. ODAC members voted 5-3 to maintain the accelerated approval of pembrolizumab for the first-line bladder indication.
References
Related Content: