Understanding The Statistics: Cancer Survival
It is important to remember that all cancer survival numbers are based on averages across huge numbers of people. These numbers cannot predict what will happen in your individual case.
Survival rates will not tell you how long you will live after you have been diagnosed with bladder cancer. But, these numbers can give you an idea of how likely your treatment will be successful. Also, survival rates take into account your age at diagnosis but not whether you have other health conditions too.
Quality Assessments Of Included Studies
Level of evidence was accessed for all 25 included studies and results were listed in Table . Among 16 RCTs, 9 were in low risk of bias, , , , , , , , , 6 were in moderate risk of bias, , ,,, and the remaining one was in high risk of bias according to the quality assessment . However, the risk of detection and attrition biases were low in all of them. Additionally, 7 RCTs were in relative high quality, , , , , , .
Figure 2
Assessment of bias risk for included RCTs. Methodological quality graph: authors judgments about each methodological quality item presented as percentages across all included studies Methodological quality summary: authors judgments about each methodological quality item for each included study, + low risk of bias ? unclear risk of bias high risk of bias).
Selecting And Testing Gemcitabine
When Dr. Messing began research on gemcitabine as a possible way to reduce recurrences more than a decade ago, the drug was not widely used for bladder cancer. “We tried to pick an agent that we thought would be safe and effective,” he said.
The researchers decided to compare gemcitabine against placebo rather than mitomycin C, based on studies showing how infrequently patients received some form of chemotherapy following surgery despite guidelines recommending this approach.
“If the new procedure were adopted widely, we could spare patients a lot of suffering from repeated surgeries and save health care costs associated with those surgeries,” Dr. Messing said.
“Now that we have the results of the trial,” he went on, “we hope that patients and physicians will embrace this approach to treatment.”
Also Check: Physical Therapy For Bladder Leakage
What Is A Rash On The Palms And Soles
A rash primarily involving the palms, soles, and genitalia is thought to be a manifestation of a hypersensitivity reaction . Both the chemical cystitis and the rash generally respond to treatment with corticosteroids. In addition, wounds in the bladder do not heal properly once intravesical mitomycin is started.
Thermal Intravesical Chemotherapy Reduce Recurrence Rate For Non
- Department of Urology, Tianjin Medical University General Hospital, Tianjin Medical University, Tianjin, China
Background: Non-muscle invasive bladder cancer accounts for nearly 80% of newly diagnosed bladder cancer cases, which often recur and progress. This meta-analysis was evaluated by the adverse events and recurrence rate of thermal intravesical chemotherapy vs. normal temperature intravesical chemotherapy in the treatment of non-muscle invasive bladder cancer.
Methods: A systematic review and cumulative analysis of studies reporting adverse events and recurrence rate of thermal intravesical chemotherapy vs. normal temperature intravesical chemotherapy was performed through a comprehensive search of Pubmed, Embase, Cochranelibrary.com, CNKI, Wanfang Med Online database and VIP database. All analyses were performed using the Revman manager 5.
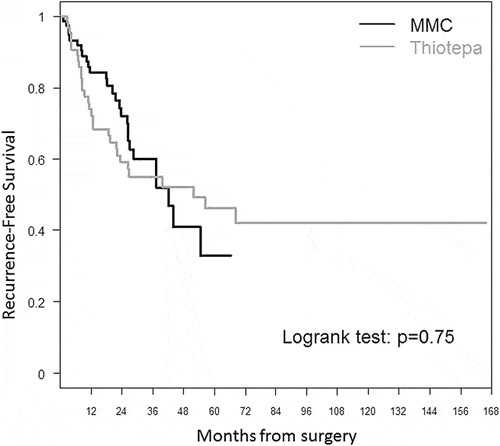
Result: Twelve studies including 888 patients, 445 in the thermal intravesical chemotherapy group, and 443 in the normal temperature intravesical chemotherapy group, met the eligibility criteria. Patients in the thermal intravesical chemotherapy group had a lower risk of disease recurrence than those who had normal temperature intravesical chemotherapy while no significant difference in adverse events rate .
Don’t Miss: Does Diabetes Cause Bladder Problems
Electromotive Intravesical Drug Administration
Electromotive drug administration uses an electric current to enhance transepithelial drug penetration . EMDA is administered via a battery-powered generator delivering an electric current of 030 mA DC at 055 V, which is passed between two electrodes. An active electrode is placed into the bladder as part of a transurethral catheter and dispersive ground electrode pads are placed on the skin of the lower abdomen .
The penetration of MMC into the bladder wall with EMDA is significantly greater than that achieved by passive diffusion as shown in in vitro studies . In clinical practice, EMDA MMC has been shown to be safe with no life-threatening adverse events. Most reports on EMDA have focussed on its role following first TURBT and there are limited reports of its use in high-risk patients.
In one such study, Di Stasi et al. reported a prospective RCT of 108 patients who were randomised into three equal groups of 36 each who underwent 40 mg electromotive MMC instillation with 20 mA electric current for 30 min, 40 mg passive MMC with a dwell time of 60 min or 81 mg BCG with a dwell time of 120 min. The complete response for electromotive versus passive MMC at 3 and 6 months was 53 versus 28 % and 58 versus 31 % . For BCG, the responses were 56 and 64 %. Median time to recurrence was 35 versus 19.5 months and for BCG it was 26 months .
How Is Mitomycin Supplied
Mitomycin for Injection USP
NDC 72819-152-95 5 mg individually-boxed amber vial.
Storage: Store dry powder at 20° to 25°C , protected from light. Avoid excessive heat, over 40 °C . Protect reconstituted solution from light. Store solution under refrigeration 2° to 8 °C , discard after 14 days. If unrefrigerated, discard after 7 days.
Read Also: Over The Counter Bladder Infection Medicine
Also Check: Bladder Cancer Metastasis To Lung Prognosis
Summary Of Main Results
This meta-analysis was conducted to synthesize the existing evidence considering the management of recurrent NMIBC using either chemoresection by MMC or surgical treatment by TURBT.
After literature searching, we identified three studies that fulfilled the inclusion criteria of this meta-analysis.810 Other studies have assessed the use of MMC for chemoresection, but they did not include a comparative group for patients who underwent TURBT.
There were some variations in the inclusion criteria of the studies included in this meta-analysis, particularly as regards the grading or risk category of patients. Racioppi et al.10 studied patients with low-grade recurrences only following the management of primary NMIBC of Ta or T1 stage. Lindgren et al.8 included patients having primary NMIBC of Ta stage with low or high grade. Mostafid et al.9 assessed patients with low grade, stage Ta NIMBIC. Moreover, there were some variations in the TURBT groups across the studies as regards the use and duration of adjuvant chemotherapy or BCG. The studies by Racioppi et al.10 and Lindgren et al.8 utilized the same dose and duration of neoadjuvant MMC , while Mostafid et al.9 administered neoadjuvant MMC for a longer duration . These variations are expected to contribute to the heterogeneity of results across the studies, particularly those of complete response.
Risk Adjusted Surveillance And Follow
Guideline Statement 32
32. After completion of the initial evaluation and treatment of a patient with NMIBC, a clinician should perform the first surveillance cystoscopy within three to four months.
Discussion
The natural history of NMIBC is often characterized by recurrence, even for solitary, small, low-grade papillary tumors. At the time of first evaluation and treatment, none of the existent risk stratification tools or urinary biomarkers is sufficiently sensitive and specific to predict which patient will have an early tumor recurrence. Therefore, the only reliable way to know in a particular patient whether they are at risk for early recurrence is by cystoscopic visualization of the urothelium at a relatively early interval after the first treatment/resection. In addition, visualization at a relatively early interval allows the treating urologist to verify that the initial resection was complete. The Panel, therefore, felt that the first repeat cystoscopic evaluation should occur three to four months after the initial treatment and evaluation, regardless of the patient’s overall risk.
Guideline Statement 33
33. For a low-risk patient whose first surveillance cystoscopy is negative for tumor, a clinician should perform subsequent surveillance cystoscopy six to nine months later, and then annually thereafter surveillance after five years in the absence of recurrence should be based on shared-decision making between the patient and clinician.
Discussion
Discussion
Recommended Reading: Overactive Bladder After Uti Treatment
When To Contact Your Team
Your doctor, nurse or pharmacist will go through the possible side effects. They will monitor you closely during treatment and check how you are at your appointments. Contact your advice line as soon as possible if:
- you have severe side effects
- your side effects arent getting any better
- your side effects are getting worse
Early treatment can help manage side effects better.
We haven’t listed all the side effects here. Remember it is very unlikely that you will have all of these side effects, but you might have some of them at the same time.
The side effects of mitomycin C given into the bloodstream are listed below.
Agreements And Disagreements With Other Studies Or Reviews
Up to the best of the authors knowledge, this is the first meta-analysis to compare the efficacy and safety of chemoresection with MMC and TURBT in patients with recurrent NMIBC. A systematic review by Alsyouf et al.23 addressed the potential efficacy of several chemoablative agents including MMC. However, they did not critically appraise the included studies or conducted a quantitative analysis and synthesis of results. A recent meta-analysis by Li et al.24 has compared the efficacy and safety of intravesical MMC and gemcitabine following TURBT to prevent recurrences. They reported that gemcitabine was superior to MMC in terms of reduction of the recurrence rate and local adverse effects.
Also Check: Does Azo Help Bladder Spasms
Intravesical Immunotherapy With Bcg
BCG is a live attenuated strain of Mycobacterium bovis, originally developed as a vaccine for tuberculosis. There are several strains available from different manufacturers with the commonest used in the UK being Oncotice BCG®. The content of a vial is reconstituted with 50 ml of saline and then administered through a urethral catheter to remain in the urinary bladder for about 2 h time. The mechanism of action is incompletely understood but it is postulated to work via a local immune response characterised by induced expression of cytokines in the urine and bladder wall . Before starting BCG in new patients, it is important to adequately debulk all visible tumour and re-resect T1 high grade tumours to reduce understaging. BCG strain may have an impact on treatment outcome in NMIBC immunotherapy. A recent prospective randomised study of 142 high-risk NMIBC patients with two of the commonest BCG strains showed that treatment with BCG Connaught conferred significantly greater 5-year recurrence-free survival compared with treatment with BCG Tice .
Table 1 Options in BCG shortage
Bladder Cancer Stages And Survival Rates
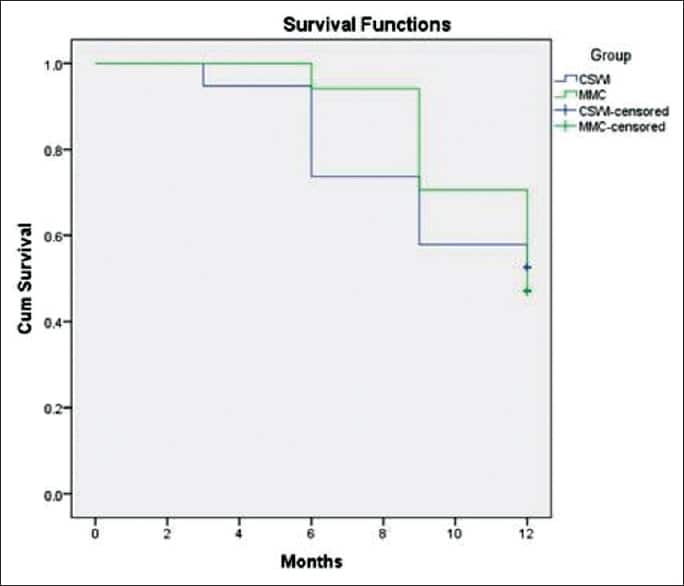
Cancer survival rates are also categorized according to the stage of the cancer when it was diagnosed. The stage of cancer generally refers to how far it has progressed, and whether it has spread to other parts of the body. For bladder cancer, the 5-year survival rate for people with:2,3
- Bladder cancer in situ is around 96 percent
- Localized bladder cancer is around 70 percent
- Bladder cancer that has spread to the regional lymph nodes is 35 percent
- Distant or metastasized bladder cancer is 5 percent
If you would like to learn more about bladder cancer statistics, consider speaking with someone on your health care team. They will be able to explain more about how these statistics apply to your cancer. Tell us about your experience in the comments below, or with the community.
Read Also: Antibiotics Used For Bladder Infections
Results Of The Search
The literature search yielded a total of 221 articles. The titles and abstracts of 158 articles were screened after the removal of duplicates, with subsequent exclusion of 180 articles, which were not relevant to the research question of the systematic review. The full text of the remaining eight articles was retrieved and assessed for eligibility. Out of the examined eight full-text articles, three studies were eligible for inclusion .810
PRISMA flow diagram
Inclusion And Exclusion Criteria
The study inclusion criteria were shown as follows: randomized controlled trials as well as quasi-RCTs studies that comprised medium- to high-risk patients who occupies 1544% NMIBC cases in certain series studies that mentioned clinical outcomes, Ta or T1 tumor and included patients receiving intravesical gemcitabine comparing with mitomycin.
The study exclusion criteria were as follows: non-RCTs studies in which cases with other neoplasm studies with incomplete information to analysis duplicate articles.
Read Also: Is Better Bladder Fda Approved
Efficacy Of Bladder Intravesical Chemotherapy With Three Drugs For Preventing Non
Zhixin Ling
Abstract
1. Introduction
Bladder cancer has the clinical characteristics of high recurrence rate, high progression rate, and high mortality rate. Most of it comes from epithelial tissue. The urothelial cell carcinoma accounts for nearly 90% of bladder cancer worldwide . According to the extent of cancer cells invading the bladder wall, non-muscle-invasive bladder cancer and muscle-invasive bladder cancer are the two main types of bladder cancer, and non-muscle-invasive bladder cancer is among the most common types of bladder cancer. The non-muscle-invasive bladder cancer is responsible for 75%85% of newly diagnosed cases .
2. Materials and Methods
2.1. Research Object
This study retrospectively analyzed a total of 335 intermediate- and high-risk patients who underwent transurethral bladder tumor resection in our hospital from October 2015 to October 2019, and they were regularly perfused with epirubicin, gemcitabine, and pirarubicin. The risk of patients was classified with intermediate- or high-risk non-muscle-invasive bladder cancer. According to the different perfusion drugs, the patients were divided into an epirubicin group , gemcitabine group , and pirarubicin group .
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Surgical Plan
2.3. Perfusion Scheme
2.4. Follow-Up
2.5. Observation Indicators
2.6. Data Analysis
3. Results
3.1. Comparison of General Information
4. Discussion
Data Availability
Conflicts of Interest
Acknowledgments
Increased Risk Of Infection
Increased risk of getting an infection is due to a drop in white blood cells. Symptoms include a change in temperature, aching muscles, headaches, feeling cold and shivery and generally unwell. You might have other symptoms depending on where the infection is.
Infections can sometimes be life threatening. You should contact your advice line urgently if you think you have an infection.
Also Check: Medicine To Help Empty Bladder
What Is The Evidence Base For This Information
This leaflet includes advice from consensus panels, the British Association of Urological Surgeons, the Department of Health and evidence based sources it is, therefore, a reflection of best practice in the UK. It is intended to supplement any advice you may already have been given by your urologist or nurse specialist as well as the surgical team at Addenbrookes. Alternative treatments are outlined below and can be discussed in more detail with your urologist or specialist nurse.
Instillation Regimens And Prognoses Of Intravesical Mmc Plus Bcg
1361 NMIBC patients from 25 eligible studies received intravesical MMC plus BCG instillation as an adjuvant therapy besides surgery . Combination regimens in these studies could be divided into four subtypes: single dose of perioperative MMC prior to BCG was applied in 4 studies, , , sequential instillations with MMC and BCG were used in 12 studies, ,,,,,,,, ,, 7 studies, ,,,, , adopted alternating instillations with MMC and BCG and last 2 studies, preferred mixed instillations with MMC plus BCG . Table showed prognoses of patients receiving combination therapies in all included studies according to different instillation regimen and follow-up time.
Table 2 Detailed outcomes of patients receiving combination therapy.
Recommended Reading: Lemon Juice For Bladder Infection
Study Search And Selection
Eligible studies focusing on the topic were identified through searching Pubmed, Embase, Cochranelibrary.com, CNKI, Wanfang Med Online database and VIP database. The search strategy is given in Appendix I. We also browsed reference lists of systematic reviews on this topic to find any other qualified articles. All searches without language limits but limited to studies on humans.
Two independent reviewers examined the titles and abstracts according to eligibility criteria mentioned before. Studies underwent full-text examination after removing duplicated, irrelevant, review, case report, letter, editorial and non-comparative design studies. Divergences were resolved by discussion with another reviewer .
What Is Intravesical Therapy To Treat Bladder Cancer
Therefore, the total effective rate was 76 per cent. We concluded from our studies that mitomycin C topical therapy could be applied to low stage and low grade bladder tumors less than 1 cm. in diameter, without regard to the number of tumors. We now use mitomycin C topical therapy in all cases of bladder tumors.
Recommended Reading: Can A Ct Urogram Detect Bladder Cancer
How Is Mitomycin Given
Mitomycin is given as an infusion into a vein. A healthcare provider will give you mitomycin.
Tell your caregivers if you feel any burning, pain, or swelling around the IV needle when mitomycin is injected.
Some people receiving mitomycin have developed ulcers on the skin where an injection was given, or on other areas of body. Skin changes may occur several weeks or months after a mitomycin injection.
Mitomycin affects your immune system. You may get infections more easily. You will need frequent medical tests, and your next dose may be delayed based on the results.
Mitomycin can have long lasting effects on your body. You may also need medical tests for a short time after your last dose.
Significantly Lower Recurrence Risk Seen
The recurrence risk in the entire cohort was significantly lower at 27% in the immediate instillation group compared to 36% in the delayed instillation group . Further, the difference in time to recurrence after 3 years of follow-up significantly favored an immediate instillation, with 34% reduction in the relative risk of recurrence compared to delayed instillation. The 3-year cancer progression rate was lower with immediate instillation compared to delayed instillation . However, the trial was not powered or designed to evaluate the risk of progression.
When analyzing each risk group separately, no difference was noted in the risk of recurrence in the LR group from immediate versus delayed instillation . However, immediate instillation significantly reduced the risk of recurrence in both the IR and HR group .
Read: ADT plus salvage RT lowers PCa metastases, raises survival
Adverse effects were recorded in 258 of 1,048 patients in the immediate instillation group and 257 of 1,195 patients in the delayed instillation group . Most common adverse effects were skin rash and irritative voiding symptoms . In six patients in the immediate instillation group, MMC extravasation was reported, which was managed conservatively.
It appears that for all NMIBC patients, an immediate single instillation of MMC within 24 hours after TURBT reduces the recurrence rate and prolongs time to recurrence, regardless of whether adjuvant MMC instillations were given.
Also Check: Medicine For Bladder Infection Pain