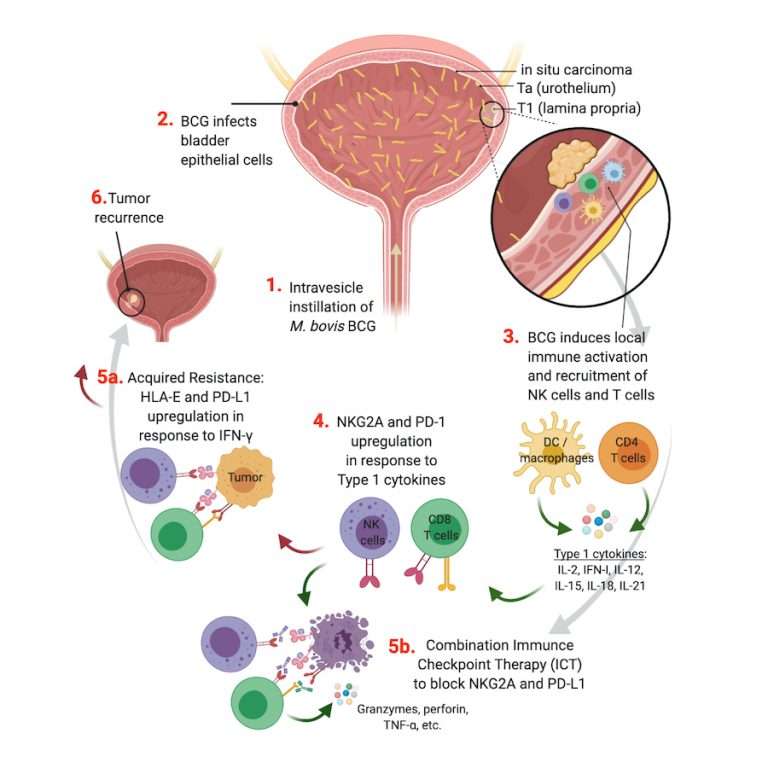
Bdd Can Be Exclusively Eliminated Via Renal Clearance
Bdd was designed in D-configuration to avoid degradation by protease enzymes in the blood circulation . It was composed of multiple D-aspartic acid and -alanine residues . The aspartic acids contributed to the overall negative charge, preventing nonspecific uptake by major organs, and promoting renal clearance of the peptide . The B residues served as linkers to avoid the formation of a secondary structure. To investigate how the charges were critical for governing Bdd’s in vivo behavior, we also synthesized a panel of Bdd analogues that were in L-configuration , neutral , carrying positive charges , and in pegylated form for comparative studies. The peptides were labeled with 89Zircronium , a long-lived radioisotope , which allowed us to study the PKs and long-term biodistribution using PET/CT imaging. The radiolabeled peptides were synthesized by first conjugating a deferoxamine chelator to the peptide in solid phase after amino acid elongation. We then complexed 89Zr with the resulting DFO-peptide conjugates in solution under basic conditions .
Study Drug Dose And Dwell Time
When trial outcomes were analyzed by drug, gemcitabine and interferon -2b did not show a benefit on recurrence, whereas the other 5 drugs did . Because epirubicin and mitomycin were administered to most of the patients in the trials, a separate random-effects model was performed on this subset. The RR for recurrence was 0.71 , which was similar to that of the overall analysis. The dwell time of the drugs ranged from 25 to 120 minutes, with 60 minutes being the most commonly reported duration of therapy. Drug doses and concentrations varied for thiotepa, epirubicin,
Study Characteristics
Risk Adjusted Surveillance And Follow
Guideline Statement 32
32. After completion of the initial evaluation and treatment of a patient with NMIBC, a clinician should perform the first surveillance cystoscopy within three to four months.
Discussion
The natural history of NMIBC is often characterized by recurrence, even for solitary, small, low-grade papillary tumors. At the time of first evaluation and treatment, none of the existent risk stratification tools or urinary biomarkers is sufficiently sensitive and specific to predict which patient will have an early tumor recurrence. Therefore, the only reliable way to know in a particular patient whether they are at risk for early recurrence is by cystoscopic visualization of the urothelium at a relatively early interval after the first treatment/resection. In addition, visualization at a relatively early interval allows the treating urologist to verify that the initial resection was complete. The Panel, therefore, felt that the first repeat cystoscopic evaluation should occur three to four months after the initial treatment and evaluation, regardless of the patient’s overall risk.
Guideline Statement 33
33. For a low-risk patient whose first surveillance cystoscopy is negative for tumor, a clinician should perform subsequent surveillance cystoscopy six to nine months later, and then annually thereafter surveillance after five years in the absence of recurrence should be based on shared-decision making between the patient and clinician.
Discussion
Discussion
Recommended Reading: What Are The Side Effects Of Botox In The Bladder
Synthesis Of Cleavable Drugpeptide Conjugates
DM1 was added to SPDP-peptide in a cosolvent of NMP and PBS , and allowed to react for 2 days at room temperature. The DM1-peptide was then purified by rp-HPLC. To conjugate aldox, a thiol-reactive side was introduced at the peptide N-terminal. Aldox was added to the peptide in PBS , and allowed to react for 30 minutes. The drugpeptide obtained was pH-sensitive and thereby was purified by rp-HPLC in neutral conditions . Size exclusion chromatography was used to remove the salt content. All the final drugpeptide conjugates were characterized using MALDI-TOF analysis and were quantified with UV absorbance, according to the predetermined extinction coefficient of DM1 or aldox in 5% PBS in methanol.
When Is Intravesical Chemotherapy Used
Intravesical chemotherapy is used mainly for low-to medium-risk non-muscle-invasive bladder cancer. It helps prevent the cancer coming back . This method of giving chemotherapy cant reach cancer cells outside the bladder lining or in other parts of the body, so its not suitable for muscle-invasive bladder cancer.
Also Check: What’s The Treatment For Bladder Cancer
Intravesical Gemcitabine And Docetaxel For Nonmuscle
Intravesical bacillus-Calmette Guérin is the traditional gold-standard treatment of intermediate- and high-risk nonmuscle-invasive bladder cancer however, BCG therapy is fraught with frequent drug shortages and its scarcity has prompted investigation into alternative regimens. Furthermore, more than 30% of patients will experience cancer recurrence after BCG, and these patients are left with a paucity of effective second-line therapies. Intravesical gemcitabine and docetaxel has emerged over the last few years as a promising therapeutic option for these patients.
| Figure 1. Disease setting in which GemDoce is prescribed. CIS, carcinoma in situ. |
| Figure 2. Utilization of GemDoce. |
For those interested in introducing GemDoce into their urology practices, please reach out to Dr. Packiam at vignesh-packiam@uiowa.edu or Dr. Kates at .
Intravesical Chemotherapy Plus Bacille Calmette
Review published: 2013.
Bibliographic details: Houghton BB, Chalasani V, Hayne D, Grimison P, Brown CS, Patel MI, Davis ID, Stockler MR. Intravesical chemotherapy plus bacille Calmette-Guerin in non-muscle invasive bladder cancer: a systematic review with meta-analysis. BJU International 2013 111: 977-983.
Also Check: What Does Bladder Cancer Look Like On Ultrasound
Heterogeneity Influence And Publication Bias
The overall I2 measure of between-study inconsistency was 75%. To assess whether any individual study was particularly responsible for between-study heterogeneity in this pooling, the authors conducted a thorough study of influence. The leave-one-out analysis suggested that the De Nunzio et al34 study was an outlier, because the I2 decreased to 75% from 61% when it was removed. When each of the other individual 17 studies was removed, the I2 ranged from 71% to 77%, essentially unchanged from the overall pooled value of 75%, suggesting that none of the other studies was overly influential. Examination of plots of Cook’s distance, studentized residuals, and DFFITS also suggested that study pooling was sensitive to the De Nunzio study. The pooled RR for IVC increased from 0.67 when all studies were included, to 0.72 when the De Nunzio study was removed.
To examine the possibility of publication bias, the authors visually evaluated several plots, including the standard funnel plot, the Galbraith radial plot, the normal QQ plot, and the Duval-Tweedie trim-fill funnel plot. The plots suggested the existence of publication bias and this was confirmed with Egger’s test, which suggested that small trials in the analysis disproportionally contribute to the protective effect of IVC .
When Is Chemotherapy Used
Systemic chemo can be used :
- Before surgery to try to shrink a tumor so that it’s easier to remove and to help lower the chance the cancer will come back. Giving chemo before surgery is called neoadjuvant therapy.
- After surgery . This is called adjuvant therapy. The goal of adjuvant therapy is to kill any cancer cells that may remain after other treatments. This can lower the chance that the cancer will come back later.
- In people getting radiation therapy, to help the radiation work better.
- As the main treatment for bladder cancers that have spread to distant parts of the body.
You May Like: Botox For Bladder Control Reviews
Bcg Relapse And Salvage Regimens
Guideline Statement 22
22. In an intermediate- or high-risk patient with persistent or recurrent disease or positive cytology following intravesical therapy, a clinician should consider performing prostatic urethral biopsy and an upper tract evaluation prior to administration of additional intravesical therapy.
Discussion
Urothelial carcinoma, particularly CIS, is considered a field-change disease with the entire urothelium at risk in affected individuals. Clinicians should remain aware of sites outside the bladder as potential sources for metachronous tumors. While the initial diagnostic evaluation includes radiographic/endoscopic visualization of the entire urinary tract, the extra-vesical urothelium remains at long-term risk for subsequent tumor development. Moreover, these sites may harbor disease and contribute to cancer recurrence within the bladder.
Of note, the Panel recognizes that evaluation of the upper urinary tract and urethra may be withheld in select patients who have received a single induction course of intravesical BCG and subsequently have persistent evidence of disease and are to undergo a second course of BCG.
Guideline Statement 23
23. In an intermediate- or high-risk patient with persistent or recurrent Ta or CIS disease after a single course of induction intravesical BCG, a clinician should offer a second course of BCG.
Discussion
Guideline Statement 24
Discussion
Guideline Statement 25
Discussion
Guideline Statement 26
Gemcitabine And Associated Combinations
4.3.1 Gemcitabine as a sole agent
Gemcitabine has been studied in BCG refractory cases in two trials. Lorenzo et al. conducted a multi-center prospective randomized phase 2 trial in which 80 patients failing one course of BCG were randomly allocated to Gemcitabine arm and 2nd course of BCG arm. Kaplan Meier statistics of 2-year recurrence-free survival showed a significant difference between the gemcitabine and BCG group . Seven of 21 patients in gemcitabine group and 13 of 35 patients in group had disease progression and underwent radical cystectomy . No significant safety concern was seen in both groups. The authors concluded that gemcitabine might be considered a second-line treatment after BCG failure in a high-risk non-muscle-invasive group .
Addeo et al. conducted a phase III randomized control trial in 120 high-risk NMIBC patients previously treated with BCG from march 2003 to November 2005. They received 40 mg of mitomycin C or 2000 mg of gemcitabine diluted in 50 mL of normal saline. The median follow-up was 36 months. In the gemcitabine arm, 39 of 54 patients remained free of recurrence versus 33 of 55 in the mitomycin C arm. Progression was seen in 10 patients in the mitomycin C arm and 6 in the gemcitabine arm. The incidence of chemical cystitis in the mitomycin C arm was statistically higher than in the gemcitabine arm .
4.3.2 Gemcitabine plus cabazitaxel plus cisplatin
The dosing used in this study was:
4.3.3 Gemcitabine plus mitomycin
You May Like: Does Amoxicillin Treat Bladder Infections
Sex After Intravesical Chemotherapy
Men should use a condom during sex for the first 48 hours after chemotherapy. If you are a woman having chemotherapy, your partner should use a condom during this time. This protects your partner from any of the drug that may be present in semen or vaginal fluid. Your doctor or specialist nurse can give you more information about this.
Is This Guidance Up To Date
Next review: January 2022
Coding and clinical classification codes for this guidance
This guidance replaces NICE interventional procedures guidance on electrically-stimulated intravesical chemotherapy for superficial bladder cancer .
Your responsibility
This guidance represents the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, healthcare professionals are expected to take this guidance fully into account, and specifically any special arrangements relating to the introduction of new interventional procedures. The guidance does not override the individual responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or guardian or carer.
All problems related to a medicine or medical device used for treatment or in a procedure should be reported to the Medicines and Healthcare products Regulatory Agency using the Yellow Card Scheme.
Recommended Reading: Chronic Bladder Infection In Men
Section : Clinical Questions Related To The Intravesical Bcg Immunotherapy
Question 13: What are the contraindications of intravesical BCG immunotherapy?
Recommendation: Intravesical BCG immunotherapy is contraindicated to patients with visible haematuria, symptomatic urinary tract infection, recent history of traumatic catheterization, active tuberculosis, severe immunosuppression , allergy to BCG, and operations within two weeks of TURBT. .
Evidence summary: We referred to the recommendations from the EAU guideline , NCCN guideline , and Guidelines for Diagnosis and Treatment of Urology and Andrology in China .
Question 14: Is intravesical BCG immunotherapy prior to intravesical chemotherapy in patients with NMIBC?
Recommendations: For patients with high-risk tumors, intravesical BCG immunotherapy is recommended. . For patients with intermediate-risk tumors, intravesical chemotherapy or intravesical BCG immunotherapy is recommended.
Implementation consideration: Treatment schemes for intravesical BCG immunotherapy: starting intravesical BCG instillation within 24 weeks after TURBT The patients should first be given BCG induction instillation for 68 weeks , followed by BCG maintenance instillation for 13 years , and then repeat the treatment every 6 months .
Question 15: Is a standard dose of BCG immunotherapy superior to a low dose of BCG immunotherapy for the patients with intermediate-risk and high-risk NMIBC?
Question 18: What is the treatment option after the intravesical BCG immunotherapy failed?
Table 2 Management of BCG side effects
How Is Chemo Used To Treat Bladder Cancer
Intravesical chemotherapy is typically used to treat patients with early-stage bladder cancer.1,2 Bladder cancer cells generally start to grow in the urothelium, which is the thin layer of cells that line the inside of the bladder. In early-stage bladder cancer, the cancer cells are only located in the bladder lining and have not grown into the muscle of the bladder wall.
Because it is delivered directly into the bladder, intravesical chemotherapy treatment is only effective against cancer cells located in the bladder lining. This type of treatment does not work against cancer cells that have grown deep into the bladder wall muscle or against cancer cells that are growing outside of the bladder in other organs or tissues.
Recommended Reading: Does Medicare Cover Botox Injections For Bladder
S To Incorporate Different Functionalities To Peptide
Cyanine5.5 NHS ester in DMF , DFO in DMSO , or SPDP in NMP , were added to the resin and allowed to react overnight at room temperature. For fluorophore and chelator conjugation, the reactions were performed in the presence of an organic base . The peptides were then removed from the resin using a cleaving cocktail containing TFA/thioanisole/1,2-ethandithiol/anisole for 4 hours and precipitated in methyl-tert-butyl ether. The resulting peptides were purified to > 98% purity using reverse-phase high-performance liquid chromatography and were characterized by MALDI-TOF analysis to confirm their molecular weights.
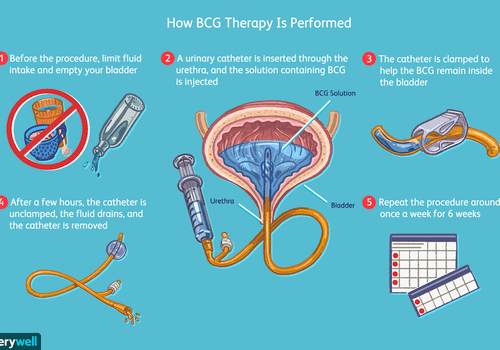
Intravesical Therapy For Bladder Cancer
With intravesical therapy, the doctor puts a liquid drug right into your bladder rather than giving it by mouth or injecting it into your blood. The drug is put in through a soft catheter that’s put into your bladder through your urethra. The drug stays in your bladder for up to 2 hours. This way, the drug can affect the cells lining the inside of your bladder without having major effects on other parts of your body.
Also Check: Stress Incontinence Vs Overactive Bladder
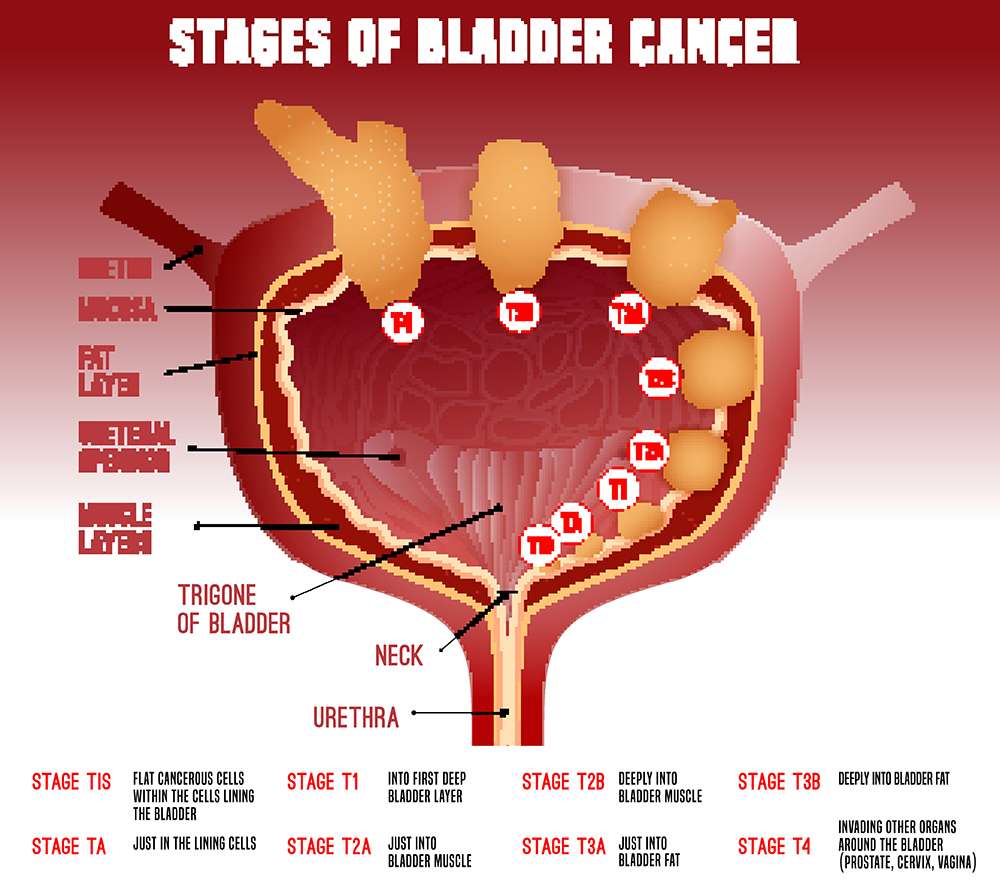
Nonmuscle Invasive Bladder Cancer
High-grade or T1 disease:
-
For T1 tumors, TURBT alone is generally not adequate treatment use of intravesical bacillus Calmette-Guerin after TURBT is recommended
Intravesical adjuvant immunotherapy for nonmuscle-invasive bladder cancer :
-
BCG 81 mg or 50 mg in 50 mL sterile saline instilled into the bladder through a catheter and held for 2 h instilled weekly for 6 wk
-
Maintenance therapy: 81 mg intravesically given on days 1, 8, and 15 of months 3, 6, 12, 18, 24, and 36 after initiation
BCG-unresponsive nonmuscle-invasive bladder cancer:
- Pembrolizumab is indicated for treatment of BCG-unresponsive, high-risk NMIBC with carcinoma in situ with or without papillary tumors in patients who are ineligible for, or have elected not to undergo, cystectomy.
- Pembrolizumab 200 mg IV q3wk until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression
- Valrubicin is indicated for intravesical therapy of BCG-refractory CIS of the urinary bladder in patients for whom immediate cystectomy would be associated with unacceptable morbidity or mortality.
- Valrubicin 800 mg added to 55 mL sterile saline instilled into the bladder through a catheter and held for 2 h instilled weekly for 6 wk
Use Of Intravesical Chemotherapy Based On Clinical Scenario
Immediate post-operative instillation
Transurethral resection of bladder tumor is the mainstay of both diagnosis and treatment of NMIBC, and provides both the stage and the basis of risk stratification. Despite visually complete resection, there is a high rate of recurrence in patients with NMIBC, possibly due to implantation of tumor cells after TURBT or residual but not visible disease. Sylvester et al. performed a comprehensive meta-analysis compiling clinical trials examining almost 1500 patients and found a reduction in recurrence rate from 48.4% to 36.7% in patients receiving immediate instillation of intravesical chemotherapy . MMC, doxorubicin, and epirubicin showed benefit however, epirubicin is not currently available in the United States. This benefit appears to be greatest in solitary, low-volume tumors. Intravesical gemcitabine does not have a role in this setting, as a phase 3 randomized controlled trial performed on over 350 patients did not show benefit as was seen for other agents, when compared to placebo .
Adjuvant instillation of intravesical chemotherapy
You May Like: What Causes Bladder Leakage When Coughing
Quality Assessment And Data Extraction
The quality of all included RCTs was assessed using the risk of bias tool recommended by the Cochrane Collaboration. It consists of the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias. The Newcastle-ottawa quality scale was used to assess the quality of retrospective studies. Two reviewers independently evaluated the quality of studies in these domains.
Data extraction was also executed by two reviewers independently. The following information was extracted: first author’s name, year of publication, study period, study design, sample size, clinical protocols, and number of patients who completed the study, age of participants, median follow-up, treatment schedule, and relevant data on outcomes. Disagreements were discussed and consensus was finally achieved.
Thermal Intravesical Chemotherapy Reduce Recurrence Rate For Non
- Department of Urology, Tianjin Medical University General Hospital, Tianjin Medical University, Tianjin, China
Background: Non-muscle invasive bladder cancer accounts for nearly 80% of newly diagnosed bladder cancer cases, which often recur and progress. This meta-analysis was evaluated by the adverse events and recurrence rate of thermal intravesical chemotherapy vs. normal temperature intravesical chemotherapy in the treatment of non-muscle invasive bladder cancer.
Methods: A systematic review and cumulative analysis of studies reporting adverse events and recurrence rate of thermal intravesical chemotherapy vs. normal temperature intravesical chemotherapy was performed through a comprehensive search of Pubmed, Embase, Cochranelibrary.com, CNKI, Wanfang Med Online database and VIP database. All analyses were performed using the Revman manager 5.
Result: Twelve studies including 888 patients, 445 in the thermal intravesical chemotherapy group, and 443 in the normal temperature intravesical chemotherapy group, met the eligibility criteria. Patients in the thermal intravesical chemotherapy group had a lower risk of disease recurrence than those who had normal temperature intravesical chemotherapy while no significant difference in adverse events rate .
Don’t Miss: Fastest Way To Cure Bladder Infection