Immunotherapy For Bcg Unresponsive Nmibc: Future Directions
Immunotherapy could be the mainstream treatment option or one of the standard treatment options not only for MIBC, but also for BCG unresponsive NMIBC, if the results of the current ongoing trials, which are expected in a few years, are promising. BCG naive NMIBC could serve as the other target for immunotherapy in the near future, although some biomarkers are necessary for selecting the appropriate treatment targets for optimizing the individualized treatment options for patients with bladder cancer. In addition, due to the world-wide BCG shortage, the novel treatment methods with reliable effects for BCG naive NMIBC is desperately awaited.
Fda Approves Mercks Keytruda For Patients With Bcg
KEYTRUDA Is the First Anti-PD-1 Therapy Approved for Certain Patients With High-Risk, Non-Muscle Invasive Bladder Cancer
KENILWORTH, N.J.Merck , known as MSD outside the United States and Canada, today announced that the U.S. Food and Drug Administration has approved KEYTRUDA, Mercks anti-PD-1 therapy, as monotherapy for the treatment of patients with Bacillus Calmette-Guerin -unresponsive, high-risk, non-muscle invasive bladder cancer with carcinoma in situ with or without papillary tumors who are ineligible for or have elected not to undergo cystectomy.
Todays approval of KEYTRUDA reinforces our companys commitment to expanding existing treatment options for certain patients with high-risk, non-muscle invasive bladder cancer, said Dr. Scot Ebbinghaus, vice president, clinical research, Merck Research Laboratories. As the first anti-PD-1 therapy approved in this setting, KEYTRUDA will be a new clinical option for a patient population that previously had limited FDA-approved therapies available.
Data Supporting the Approval
Patients received KEYTRUDA 200 mg every three weeks until unacceptable toxicity, persistent or recurrent high-risk NMIBC, or progressive disease. Assessment of tumor status was performed every 12 weeks for two years and then every 24 weeks for three years, and patients without disease progression could be treated for up to 24 months. The major efficacy outcome measures were complete response and duration of response.
Melanoma
Gastric Cancer
Cg0070 Plus Pembrolizumab Yields Positive Preliminary Data In Non
Patients with non-muscle invasive bladder cancer unresponsive to Bacillus Calmette-Guerin may benefit from treatment with CG0070 plus pembrolizumab.
Treatment with CG0070 and pembrolizumab resulted in promising preliminary findings in a small population of patients with non-muscle invasive bladder cancer who were unresponsive to Bacillus Calmette-Guerin , according to a press release on the phase 2 CORE1 trial .
The preliminary findings indicated that the combination was well-tolerated with promising efficacy in the studys population of 9 patients. As of the data cut off in November 2021 and at the initial 3-month timepoint, investigators reported that all patients achieved a complete response . All patients who reached additional timepoints maintained a CR at 6 months and 3 maintained a CR at 9 months.
These preliminary results are exciting, Roger Li, MD, lead study investigator and urologic oncologist at Moffitt Cancer Center, said in a press release. If similar trends hold moving forward, we may have a game changer to combat BCG-unresponsive bladder cancer for patients with significant unmet medical need.
The open label trial has an estimated enrollment of 37 patients. The studys primary end point is CR with key secondary end points including safety, duration of response, overall survival, and progression-free survival.
Also Check: Chronic Bladder Infection In Men
Pembrolizumab Use In Bladder Cancer: A Tale Of Two Trials
volume 18, pages 577578
The treatment of patients with the PD1 inhibitor pembrolizumab yields benefit in the second-line metastatic urothelial cancer setting. Two new trials have studied pembrolizumab monotherapy in other patients with urothelial carcinoma: an open-label phase II trial has enrolled patients with high-risk BCG-refractory non-muscle-invasive disease, and a phase III trial has compared three first-line treatment options for patients with metastatic disease. However, these trials have shown conflicting results.
Refers to Powles, T. et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma : a randomised, open-label, phase 3 trial. Lancet Oncol.22, 931945 | Balar, A. V. et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG : an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol.22, 919930 .
This is a preview of subscription content, access via your institution
Statistical Measures And Analysis
This protocol aims at assessing the activity of one treatment arm, BCG in combination with pembrolizumab in patients with NMIBC. We would like to detect an activity which is at least 25% greater than that of historical controls from case series of BCG-only treatment.
Sample size calculation for this protocol is based on the work by Kahn et al. Using a one-stage design, 15 patients will be enrolled in this study. Patient-related data will be collected and transferred to an excel spreadsheet for statistical analysis. SAS software V.9.4 will be used for all data analysis. Descriptive statistics will be computed for all study variables. Continuous variables will be described with measures of central tendency and dispersion . Categorical variables will be summarised as frequencies and percentages. Some examples include tumour stage , age, Eastern cooperative group performance status scale , sex and use of tobacco and alcohol.
For the secondary objective, subjects will be assessed at 19 weeks, 3, 12 and 24 months post-treatment completion for complete response. The outcome end point will be complete response status at 19 weeks, 3, 12 and 24 months post-treatment to measure complete response, recorded as yes or no and summarised as proportions and a per cent.
Don’t Miss: How Do You Check For Bladder Cancer
Risk Adjusted Surveillance And Follow
Guideline Statement 32
32. After completion of the initial evaluation and treatment of a patient with NMIBC, a clinician should perform the first surveillance cystoscopy within three to four months.
Discussion
The natural history of NMIBC is often characterized by recurrence, even for solitary, small, low-grade papillary tumors. At the time of first evaluation and treatment, none of the existent risk stratification tools or urinary biomarkers is sufficiently sensitive and specific to predict which patient will have an early tumor recurrence. Therefore, the only reliable way to know in a particular patient whether they are at risk for early recurrence is by cystoscopic visualization of the urothelium at a relatively early interval after the first treatment/resection. In addition, visualization at a relatively early interval allows the treating urologist to verify that the initial resection was complete. The Panel, therefore, felt that the first repeat cystoscopic evaluation should occur three to four months after the initial treatment and evaluation, regardless of the patient’s overall risk.
Guideline Statement 33
33. For a low-risk patient whose first surveillance cystoscopy is negative for tumor, a clinician should perform subsequent surveillance cystoscopy six to nine months later, and then annually thereafter surveillance after five years in the absence of recurrence should be based on shared-decision making between the patient and clinician.
Discussion
Discussion
Pembrolizumab And Beyond For Bcg
It has now been over a year since pembrolizumab received FDA approval on January 8, 2020, for treatment for Bacillus Calmette-Guerin -unresponsive, high-risk, non-muscle invasive bladder cancer with carcinoma in situ with or without papillary tumors who are ineligible for or who have elected to not undergo radical cystectomy. This approval was a nice step towards expanding treatment options for a patient population that harbors a clear unmet medical need. Intravesical BCG is the standard for intermediate and high-risk disease, but BCG is not adequate to prevent relapses or frank resistance in approximately 50% of patients.1 BCG-unresponsive disease includes both BCG-refractory and BCG-relapse populations. BCG-refractory refers to the presence of persistent high-grade cancer 6 months after the start of induction therapy or cancers that have progressed by either stage or grade 3 months after the initiation of induction therapy.2
Below, I present other ongoing trials for BCG-unresponsive non-muscle invasive bladder cancer that we should continue to accrue aggressively to. Given the fact that this is a significantly large, unmet need population, with limited treatment options if radical cystectomy is not feasible or desired, the below trials may offer more options for our patients.
Accruing Trials for Patients with BCG-Unresponsive Urothelial Cancer
Recommended Reading: Pembrolizumab Bladder Cancer First Line
Neoadjuvant Pembrolizumab Induces Survival Benefit And High Downstaging Rate In Cisplatin
Patients with cisplatin-ineligible muscle-invasive bladder cancer experienced a better rate of downstaging and improved survival benefit with neoadjuvant pembrolizumab prior to radical cystectomy vs immediate radical cystectomy.
Treatment with neoadjuvant pembrolizumab prior to radical cystectomy resulted in improved downstaging and survival in patients with cisplatin-ineligible muscle-invasive bladder cancer compared with immediate radical cystectomy , according to data from a poster presented at the Society for Urologic Oncology 23rd Annual Meeting.
In a population of 39 patients with cisplatin-ineligible MIBC who were treated with neoadjuvant pembrolizumab as part of the phase 2 PURE-01 and 313 patients who underwent IRC at Moffitt Cancer Center, overall survival was improved for the pembrolizumab group. Median OS that was not reached and 19 months, respectively .
Moreover, OS was also found to be prolonged in the propensity score matched analysis, with a median that was not reached in the pembrolizumab arm vs 21 months in the IRC arm . In fact, the IRC population experienced a worse OS in the Cox proportional hazards modeling compared with those treated with pembrolizumab. The response rate was improved in the pembrolizumab arm at 33% vs 13% in the IRC arm .
OS in the study was assessed using the Kaplan-Meyer method, with comparisons conducted using log-rank functions.
Reference
Related Content:
Definition Of Bcg Unresponsive Nmibc
For this reason, the International Bladder Cancer Group and the Genitourinary American Society of Clinical Oncology Group announced a novel, combined definition for the most aggressive subgroup of BCG failure, namely, the BCG unresponsive subgroup. According to their definition, BCG unresponsiveness is defined by the presence of persistent high-grade disease, or disease recurrence within 6 months of receiving at least two courses of intravesical instillation of BCG, after at least 5 of 6 induction doses along with at least 2 of 3 maintenance doses, or T1 high-grade disease at first evaluation following the induction of BCG alone after at least 5 of 6 induction doses. The BCG unresponsive subgroup of NMIBC specifically comprises patients with high risk of progression, for whom additional BCG therapy is unfeasible . This novel category is important for identifying what truly defines the failure of BCG therapy, which requires optimal treatment strategies following the discontinuation of BCG immunotherapy.
Also Check: Can You Live Without A Bladder
Pembrolizumab Monotherapy For Bcg
Pembrolizumab monotherapy was found to be tolerable and produced enduring responses in patients with Bacillus Calmette-Guérin -unresponsive, nonmuscle-invasive bladder cancer who were ineligible for or did not choose to undergo radical cystectomy, according to study results published in The Lancet Oncology.
Transurethral resection of bladder tumor followed by intravesical BCG immunotherapy is the current standard of care for patients with high-risk, nonmuscle-invasive bladder cancer. Despite high initial response rates, approximately 50% of these patients relapse or become resistant to BCG immunotherapy. BCG resistance is attributed to the potential activation of the programmed death-1 pathway, the study authors explained.
In the open-label, single-arm, phase 2 KEYNOTE-057 trial , the study authors sought to determine if pembrolizumab can induce clinical complete response in BCG-unresponsive, nonmuscle-invasive bladder cancer. The multicenter trial was conducted at 54 sites, including hospitals and cancer centers, in 14 countries.
Interim results from Cohort A of this trial supported the FDA approval of pembrolizumab for this patient population. The research team reported additional results from the analyses of Cohort A that included 101 patients with carcinoma in situ with or without papillary tumors, of whom 96 were evaluable for efficacy.
Reference
Ethics Confidentiality And Regulatory Details
The investigator and sponsor of the study will adhere to all applicable data privacy and confidentiality laws and regulations ). Those applicable include institutional, state and federal law. It is the responsibility of the investigator and sponsor to ensure the subject data as well as sensitive study information is handled according to applicable guidelines and laws. Appropriate authorisation and consent for use, disclosure or transfer of protected health information must be obtained.
Subjects names will not be recorded on the case report forms . Only the subject number and subjects initials will be recorded, where permitted. If the subjects name appears on any other document , it must be obliterated on the copy of the document as appropriate. Study data stored on a computer will be stored in accordance with local data protection laws. The subjects will be informed that representatives of the sponsor or its designee, institutional review board and regulatory authorities may inspect their medical records to verify the information collected, and that all personal information made available for inspection will be handled in strictest confidence and in accordance with local data protection laws.
Qualified staff of the sponsor will monitor the study according to a pre-arranged monitoring plan. Monitoring of the study will include:
Protocol deviations will be identified and recorded on a deviation log.
Also Check: How To Strengthen Bladder Naturally
Immunotherapy For Bcg Unresponsive Nmibc: Ongoing Trials
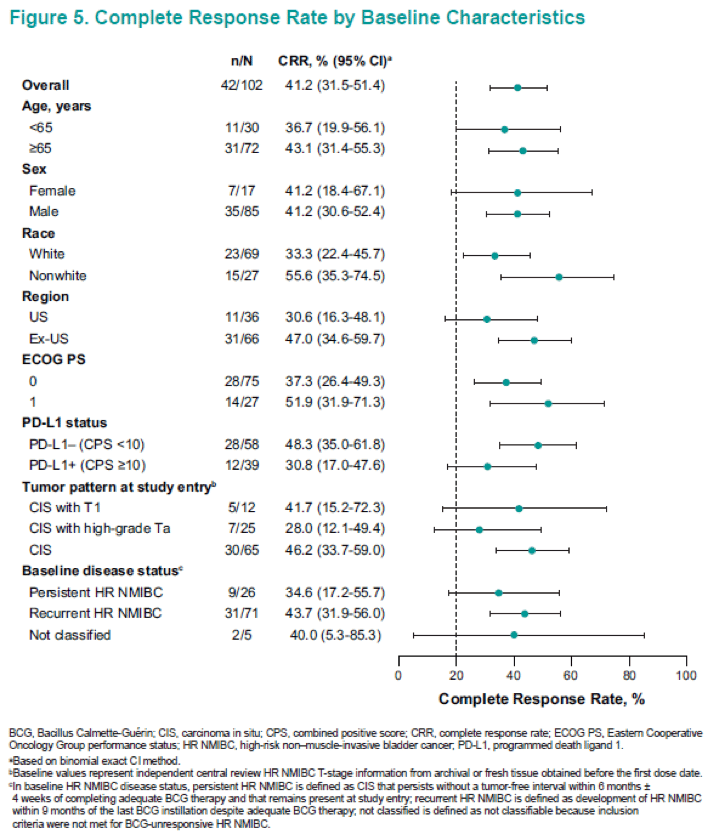
The recent preliminary results of pembrolizumab, in addition to the recently published results of the study on high-risk BCG unresponsive patients, who declined to undergo or were ineligible for cystectomy, indicated that the treatment options investigated in the studies were superior to the currently available treatment strategies. The study reported that pembrolizumab induced a complete response in nearly 40% of patients with BCG unresponsive NMIBC. Grade 34 treatment-related adverse events were observed in 12.7% of the study population, although treatment-related adverse events of any grade were observed in 64.7% of the study population. In other words, it is thought that immunotherapy is effective for treating BCG unresponsive bladder cancer, and could offer oncologically superior and safe treatment options for the treatment of these patients in the near future, although the results from phase 3 randomized clinical trials are awaited.
Role Of Immunotherapy In Bacillus Calmette
Seoul National University Boramae Medical Center , , Korea
Contributions: Conception and design: S Yoo Administrative support: S Yoo Provision of study materials or patients: S Yoo Collection and assembly of data: J Suh Data analysis and interpretation: J Suh Manuscript writing: All authors Final approval of manuscript: All authors.
Correspondence to:
Keywords: Administration intravesical Bacillus Calmette-Guérin vaccine immunotherapy urinary bladder neoplasms
Submitted Jan 29, 2020. Accepted for publication Apr 29, 2020.
doi: 10.21037/tcr-20-758
Don’t Miss: Nursing Diagnosis For Bladder Cancer
Summary Of Interim Clinical Results
Abstract #666 – Phase 2, Single Arm Study of CG0070 Combined with Pembrolizumab in Patients with Non-Muscle Invasive Bladder Cancer Unresponsive to Bacillus Calmette-Guerin
The new data for CORE1 adds to that presented at the ASCO 2022 Annual Meeting earlier this year and continues to show both promising early anti-tumor activity and tolerability of CG0070 in combination with pembrolizumab for patients with BCG-unresponsive NMIBC.
- As of the interim analysis, based on a data cutoff on October 10, 2022, 32 patients were evaluable for efficacy with a minimum of 3-month follow up.
- 88% of patients evaluable for efficacy have achieved complete response at the initial 3-month timepoint. Of those patients evaluable for CR at additional timepoints, 88% have also maintained a CR through 6 months, 86% through 9 months and 73% at the 12-month assessment.
- CG0070 in combination with pembrolizumab has been generally well tolerated with the adverse event profile consistent with that observed in prior studies of each agent alone. The most common treatment-related adverse events reported include transient grade 1-2 local genitourinary symptoms.
Role Of Cystectomy In Nmibc
Guideline Statement 27
27. In a patient with Ta low- or intermediate-risk disease, a clinician should not perform radical cystectomy until bladder-sparing modalities have failed.
Discussion
Low-grade, noninvasive tumors very rarely metastasize, and even large-volume, multifocal cancers can usually be managed with techniques, such as staged resection. Patients with low-grade recurrences can be successfully managed with intravesical chemotherapy 225 or BCG. 177,226,227 In addition, small, multifocal recurrences despite intravesical therapy can usually be treated effectively with office fulguration, repeat TURBT or even surveillance, in select cases. 64-67
Guideline Statement 28
28. In a high-risk patient who is fit for surgery with persistent high-grade T1 disease on repeat resection, or T1 tumors with associated CIS, LVI, or variant histologies, a clinician should consider offering initial radical cystectomy.
Discussion
Guideline Statement 29
29. In a high-risk patient with persistent or recurrent disease within one year following treatment with two induction cycles of BCG or BCG maintenance, a clinician should offer radical cystectomy.
Recommended Reading: How Do You Treat An Inflamed Bladder
Pembrolizumab In Bladder Cancer
- Pembrolizumab was approved for treatment of patients with Bacillus Calmette-Guérinunresponsive, high-risk, nonmuscle invasive bladder cancer with carcinoma in situ with or without papillary tumors who are ineligible for or have elected not to undergo cystectomy.
- The recommended dose of pembrolizumab in patients with high-risk BCG-unresponsive nonmuscle invasive bladder cancer is 200 mg via intravenous infusion over 30 minutes every 3 weeks until persistent or recurrent high-risk nonmuscle invasive bladder cancer, disease progression, unacceptable toxicity, or for up to 24 months in patients without disease progression.
Product labeling provides recommended dosing modifications, including withholding, resuming, and discontinuing treatment for the following adverse reactions: immune-mediated pneumonitis, colitis, endocrinopathies, nephritis, skin adverse reactions, and hepatitis in patients with and without hepatocellular carcinoma liver enzyme elevations in patients with renal cell carcinoma receiving combination therapy hematologic toxicity in patients with classical Hodgkin lymphoma or primary mediastinal large B-cell lymphoma inability to taper corticosteroid treatment persistent grade 2 or 3 adverse reactions and infusion-related reactions.
Safety Profile
REFERENCES
Background For Immunotherapy In Bcg Unresponsive Nmibc
After the first intravesical BCG clinical trial by Morales and coworkers in 1976 , Lamm and coworkers performed a prospective randomized clinical trial to confirm the effects of BCG instillation in bladder cancer . Based on these results, BCG instillation has been regarded as the standard treatment for superficial bladder cancer , and the mechanism of action of BCG via stimulation of the immune system is well established . However, around 30% of patients with NMIBC do not respond to BCG and progress towards MIBC . These results triggered an increasing interest in novel agents for the treatment of unresponsive NMIBC, and the instillation of certain chemotherapeutic agents were subsequently studied. However, it was observed that the instillation of any single chemotherapeutic agent is unable to surpass the efficacy of BCG instillation . These historical and clinical studies supported the use of other immunotherapeutic agents for the treatment of systemic bladder cancer.
Recommended Reading: How To Hold Your Bladder For A Long Time