Management Of Mesh Exposure /vaginal Extrusion
In all the cases of mesh exposure, it would be pragmatic to rule out simultaneous erosion into the urethra or bladder by cystoscopy.
Conservative management
It should be initially attempted, especially in small vaginal mesh exposure. Patient is advised to abstain from intercourse. Local application of estrogen cream might allow a layer of vaginal mucosa to grow and cover the sling. Based on patient selection, this may be helpful in 0100% cases .
Vaginal approach
It is the most preferred approach and usually performed under general or spinal anesthesia in order to have adequate exploration of mesh .
Partial removal of mesh
The extruded part of the mesh is removed and the remaining mesh is carefully examined for signs of infection. The vagina is closed with mobilized flap to cover the defect using absorbable sutures .
Partial excision of mesh extrusion by vaginal approach final appearance of vagina after mesh excision excised mesh pieces
Complete removal of mesh
Conservative mesh-preserving approach
This includes vulval pad graft coverage over the exposed mesh as recently described by Shaker et al.
Laparoscopic approach
Why Was The Use Of Mesh Paused
On July 26th 2018, at the request of the Chief Medical Officer in the Department of Health, the HSE placed a pause on the use of mesh for urinary stress incontinence and pelvic organ prolapse surgeries in Irish public hospitals, in cases where it is clinically appropriate and safe to do so. The CMO requested that the pause remains in place until the HSE completes the implementation of new recommendations.
These recommendations relate to informed consent, an accreditation system and the development of a national mesh register. This action was considered necessary to provide public assurance that the use of mesh, for these purposes, is in line with international best practice.
It is important to note that there has been no change in the evidence about the use of mesh which continues to be endorsed by all major national and international professional associations. The mesh device itself is considered safe and is approved by the relevant regulatory authority. It is widely accepted that for many women suffering the distressing symptoms of stress urinary incontinence in particular, surgical procedures using synthetic mesh devices have provided a more effective and less invasive form of treatment than traditional surgical procedures.
United States Food And Drug Administration Manufacturer And User Facility Device Experience On Use Of Vaginal Mesh In Female Pelvic Floor Reconstruction
MAUDE data represents reports of adverse events involving medical devices. The data consists of all voluntary reports since June 1993, user facility reports since 1991, distributor reports since 1993, and manufacturer reports since August 1996 and is updated on a monthly basis. There are more than 2310 complications reported with the search criteria of vaginal mesh till March 2011. The incidence of complications reported under various search criteria till March 2011 is given in Table 17. A steep increase in the incidence of reported complications with search criteria vaginal mesh and mesh erosion is noted in the MAUDE database .
Recommended Reading: What Causes Overactive Bladder In Women
Vaginal Extrusion And Erosion
One of the main concerns with bladder slings has been mesh extrusion or erosion. Extrusion and erosion both refer to mesh forcing its way into the vagina, bladder, urethra or other organ. In these cases, the mesh wears through the tissues.
According to a paper by Dr. Cristiano Mendes Gomes and colleagues, vaginal extrusion rates vary from 0 percent to 1.5 percent for retropubic slings, which are inserted through an incision in the vagina and positioned in a U shape around the urethra. The ends of retropubic slings are maneuvered between the bladder and pubic bone and brought out through incisions above the pubic bone.
For transobturator slings, the vaginal extrusion rates vary from 0 percent to 10.9 percent, according to the paper published in Internal Brazilian Journal of Urology. Known as TOT, this procedure avoids the space between the pubic bone and the bladder. Mesh is inserted through the vagina and the ends are brought out through incisions between the labia and the creases of the thighs.
Additionally, Gomes and colleagues found urethral erosion happened after less than 1 percent of sling surgeries.
Three months postoperatively, she stated that her husband felt teeth in her vagina during sexual intercourse, Siegel wrote.
A pelvic exam revealed mesh extrusion.
In some cases, conservative management of erosion may be possible. For example, some surgeons may prescribe topical estrogen cream to help vaginal tissues heal.
How Common Is The Use Of Surgical Mesh To Repair Pelvic Organ Prolapse And Stress Urinary Incontinence
About 300,000 women in the United States underwent surgery to repair POP in 2010. Surgical mesh was used in about one out of three procedures. About 250,000 women in the U.S. underwent surgery to repair SUI in 2010, with mesh placement being used in over 80% of the procedures. The mesh sling has been the most widely studied procedure for stress incontinence in the history of stress incontinence surgery. All of the major urologic and gynecologic societies in the U.S. have supported its continued use as of 2019.
Recommended Reading: Bladder Pacemaker For Urinary Retention
General Principles Of Management Of Mesh And Graft Complications
Approaches to management of mesh-related complications in pelvic floor surgery include observation, physical therapy, medications, and surgery. Table 1 presents an overview of specific mesh and graft complications and management options. There may be settings in which observation of exposed mesh is reasonable 4. Surgical intervention or referral is not always necessary for type 1 mesh exposures into the vagina. Asymptomatic exposures of monofilament macroporous meshes can be managed expectantly. For women with symptoms, a trial of vaginal estrogen can be attempted for small mesh exposures. Topical estrogen may improve or resolve the mesh exposure, though there is little prospective, comparative evidence supporting this approach. A period of 612 weeks is a reasonable period to try topical estrogen.
One multicenter study of mesh complications after reconstructive surgery found that 60% of women required two or more interventions and that the first intervention was surgical in approximately one half of cases 5. These procedures are complex and should be approached with caution. Surgeons who are unfamiliar with the original index procedure or the management issues that follow should refer the patient to a surgeon who is familiar with these types of repairs.
Mesh Erosion Lawsuits: Speak To A Lawyer
Many patients suffering from transvaginal mesh injuries wish that they had been warned of the risks and potential complications before undergoing the operation. Our product liability law team at the Willis Law Firm is committed to getting you answers and financial compensation for your medical expenses, loss of income, physical pain, and emotional suffering. Some of the transvaginal mesh products currently linked with transvaginal mesh injuries include:
- American Medical Systems: SPARC, BioArc, MiniArc, Apogee, Elevate, Monarc, In-Fast, Perigree
- Boston Scientific: AdvantageT Sling System, Prefyx Mid UT Mesh Sling System, Prefyx PPST System, Obtryx Curved Single, Obtryx Mesh Sling, Arise, Pinnacle, Solyx, Lynx
- CR Bard: Avaulta PlusT Biosynthetic Support, Avaulta SoloT Synthetic Support, Faslata Allograft, Pelvitex T Polypropylene Mesh, Pelvicol Tissue, PelviSoft Biomesh
- Johnson & Johnson: Ethicon TVT, Gynecare Prolift, Gynecare Prosima, Gynecare TVT, Gynemesh, PS
If you or a loved one is suffering from transvaginal mesh injuries from any transvaginal mesh complication please call the Willis Law Firm. We have female consultants standing by, trained to discuss sensitive issues confidentially. We are currently accepting Transvaginal Mesh Erosion & Mesh Injuries lawsuits nationwide. All cases are handled on a Contingency Fee Basis .
or Call Nationwide Toll Free 1-800-468-4878
Don’t Miss: Can A Bladder Infection Cause Dizziness
What Are Recommendations For Women Considering Surgery For Pelvic Organ Prolapse
Consider various options regarding POP treatment, including non-surgical methods and suture-based methods that donât use mesh, as they have been shown to be very effective in the long term without some of the complications associated with mesh use.
Surgery may be performed with or without the use of surgical mesh. Use of mesh may increase the risk of subsequent surgeries due to complications involving the mesh. In a small percentage of patients, additional surgeries might not solve all medical problems. However, surgical treatment with mesh may offer a more durable repair of the prolapse than non-mesh surgeries. Consult a specialist in this area as experience does help in determining the best course of management.
When Were They Introduced
The most common form of implant, called a transvaginal tape , has been widely used to treat stress incontinence across Europe, the US and Australia since the early 2000s.
Early clinical trials suggested excellent efficacy and many surgeons saw advantages over traditional open-surgery procedures, which took longer to perform, involved a longer recovery for patients and were associated with their own range of complications. By contrast, a TVT procedure typically takes 3o minutes, is performed using keyhole surgery and patients often go home the same day.
Meanwhile, the traditional treatments for pelvic organ prolapse, which included suturing to reconstruct and repair the affected organs and surrounding tissue, were proving less successful, with reports of up to 29% of women suffering another prolapse after treatment. Hysterectomy is another treatment option, which some women wish to avoid.
Because outcomes of using the mesh for incontinence and hernia were so good people were enthusiastic and confident it would also be good for prolapse, said Christopher Maher, a urogynaecologist and associate professor at the University of Queensland. Thats what the mindset was when it was introduced for prolapse around 2002.
But he said regulators around the world should have demanded more testing to ensure the mesh was as effective for treating prolapse as it was for other conditions.
Also Check: What Herbs Are Good For Bladder Control
Effect Of Infection Of Mesh Material
Contrary to the prevailing understanding of polypropylene as an inert material when used in vaginal surgeries, Clave et al., in their study of 100 explants noted that all polypropylene implants showed evidence of degradation on scanning electron microscopy after three months. Mesh damage included superficial degradation, which appeared as peeling of the fiber surface, transverse cracks in the implant threads, significant cracks with disintegrated surfaces and partially detached material, and superficial and deep flaking. Fractures were variable in number and depth. Authors described several hypotheses concerning the degradation of the polypropylene including direct oxidation, fatty acid diffusion and oxidation due to free radical attack. It was noted that polypropylene implants degraded more in the presence of an acute infection or chronic inflammation. However, none of the poly was found to be altered or degraded. Hence authors expressed a need for clinical trials to comparatively investigate the performance of new type of monofilament meshes, such as poly.
Transvaginal Mesh Removal And Repair
When transvaginal mesh kits were first approved by the FDA in 2002, the surgery was marketed as easy and effective, and it quickly became a popular choice for physicians and patients. At UT Southwestern, however, we steered clear of the procedure because it was prone to complications and its safety was never fully demonstrated by long-term research.
‘The end of transvaginal mesh surgery, as mandated by the FDA, does not mean the end of effective treatment for pelvic organ prolapse. Our team is trained in the most advanced therapies, technologies, and techniques to successfully treat POP.’
Maude Carmel, M.D.
Abouteight years ago, we began seeing more patients who had experienced complications from transvaginal mesh and wanted it removed orrepaired.
Oftentimes, the surgeon who put it in didnt know how to fix it, patients told me.
Andthats another problem: Transvaginal mesh placed for prolapse is relativelyeasy to put in, but very difficult to remove because of the way it is anchoredto bones and ligaments in the leg and pelvis. Removing all of it is nearlyimpossible.
You May Like: Bladder Ultrasound For Urinary Retention
Nerve Stimulation To Treat Overactive Bladder
Sacral nerve stimulation device implanted. Activate the pop-up dialog box.The nerves that signal the desire to pee are stimulated with tiny electrical impulses in some treatments to treat overactive bladder.
Stimulation of the sacral nerve. A tiny pacemaker-like device is implanted beneath your skin, generally in your buttock. A tiny, electrode-tipped wire attached to the device transmits electrical impulses to the sacral nerve. These painless electrical impulses stop an overactive bladder from sending information to your brain about the desire to pee.
You can attempt sacral nerve stimulation by having a wire implanted under your skin and wearing the stimulator externally. If the stimulator significantly helps your symptoms, you can have it implanted later.
The stimulator is implanted in an operating room under local anesthetic and moderate sedation as an outpatient procedure. With a hand-held programmer, your doctor can alter the degree of stimulation, and you also have control to utilize for modifications.
Stimulation of the tibial nerve. A needle is inserted into the skin around your ankle and electrical stimulation is sent from a nerve in your leg to your spine, where it links with the neurons that regulate your bladder.
Tibial nerve stimulation is done in 12 weekly sessions of around 30 minutes each. Your doctor may prescribe follow-up sessions at regular intervals to sustain the outcomes based on your reaction to the treatment.
How To Know If You Are Suffering From Vaginal Mesh Complications

Pelvic organ prolapse is an often-frustrating condition that many women experience after childbirth and with changes linked to menopause, obesity, persistent cough or constipation that strains your muscles, and other common issues.
Its caused by weakening of the pelvic floor muscles that normally hold your uterus, urinary bladder, and other pelvic organs in place. As these muscles stretch and weaken, your pelvic organs can begin to droop out of place. Though not typically life-threatening, pelvic organ prolapse can cause severe urinary incontinence , painful sexual intercourse, fecal incontinence , and other life-altering issues.
Depending on the extent of the prolapse, doctors may recommend transvaginal mesh surgery to help resolve your symptoms. The mesh is a surgical material that is implanted during the procedure and designed to reinforce the vaginal walls and supply support for your bladder and urethra. Transvaginal refers to the surgical approach and may be suggested because its minimally invasive and eliminates the need for external incisions.
Read on to learn what he has to say about the complications and symptoms you may experience after vaginal mesh surgery.
Read Also: Bcg Chemo For Bladder Cancer
Transvaginal Mesh Removal At A Glance
- Women who are suffering from pelvic organ prolapse may opt for surgery to treat serious symptoms that do not improve with lifestyle changes or other devices to help support the prolapsed organs.
- Several surgical methods of repairing pelvic floor disorders exist, including a method known as transvaginal mesh implantation.
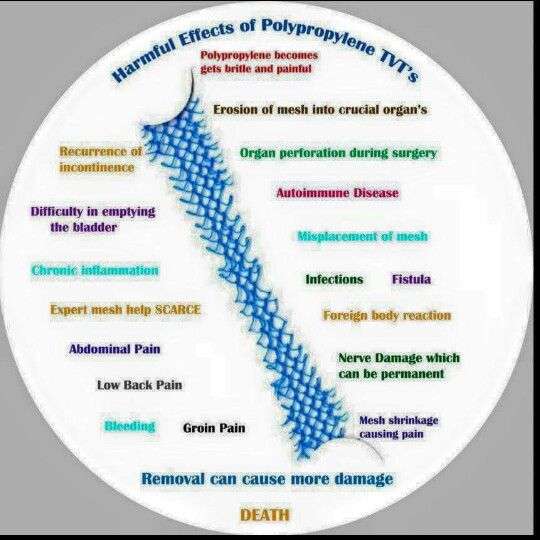
- Frequently reported complications from transvaginal mesh include chronic pain, infection, bleeding, pain during intercourse, urinary problems, and exposure of the mesh through the vagina.
- Transvaginal mesh removal is a technically complex surgical procedure in which surgeons attempt to remove as much of the mesh as possible.
- Complete transvaginal mesh removal is possible for some women, while only part of the mesh can be removed in other women due to complicated issues from the type of mesh that was originally used.
What Actions Has The Fda Taken
In 2008,the US Food and Drug Administration issued a Public Health Notification and Additional Patient Information that alerted healthcare providers and consumers about serious complications linked to the transvaginal placement of surgical mesh to treat pelvic organ prolapse and stress urinary incontinence .
The agency had received over 1,000 adverse event reports of complications arising from the use of mesh devices for POP and SUI repair. Most frequently reported complications include:
- Mesh erosion
- Dyspareunia
- Organ perforation
Years later, on July 2011, the FDA issued an update noting that serious complications associated with transvaginal mesh surgery for POP are not rare. The agency added that it is not clear whether transvaginal POP repair using mesh is more effective than traditional non-mesh repair.
Don’t Miss: What Does The Start Of A Bladder Infection Feel Like
Background And General Principles
In gynecologic surgery, grafts or mesh may be used when the surgical procedure requires the use of bridging material to reinforce native structures. The term graft refers to a biological material that comes from either a human or an animal . Autologous grafts can be harvested from the same person, whereas allografts come from human donors or cadavers. In gynecologic surgery, mesh refers to synthetic material . Because of complications attributed to multifilament and small-pore-size synthetic mesh, type 1 synthetic meshes currently are used in the United States. This document focuses on the management of complications related to mesh used to correct stress urinary incontinence or pelvic organ prolapse .
Signs And Symptoms Of Vaginal Sling Failure
The FDA released a warning in 2008 to alert women to potential vaginal sling complications, which include:
- Infection
- Bowel, bladder and blood vessel perforation during insertion
- Vaginal scarring
- Dyspareunia
It is believed that many of these problems stem from shrinkage, contraction, or erosion of the mesh.
Also Check: Bladder Infection Every Time I Have Intercourse
Complications During Or Shortly After Surgery
Intraoperative or perioperative complications occur during surgery or shortly after. In general, these are rarer. Complication rates range from less than 1 percent to 14 percent, according to Costantini and colleagues. Major complications such as vascular and nerve injuries and gut lesions occurred in less than 1 percent of women. Minor bladder injuries had rates from 0.5 to 14 percent. Significant blood loss occurred in about 2.7 percent to 3.3 percent of women.
A Briefhistory Of Mesh: Slings Vs Transvaginal
Inthe aftermath of the FDA ruling, mesh has become a four-letter word to most patients.But its important to remembernot all procedures that employ surgical mesh are created equal.
The FDA ban only applies to transvaginal mesh for prolapse repairs, which means there are still safe surgical options for treating POP and incontinence. These include:
- Bladder orvaginal slings: Theyve been around since the 1990s and are still consideredthe gold standard for treatment for stress urinary incontinence. Made of synthetic mesh or humantissue, the sling acts like a hammock around the urethra, preventing it fromopening during stresses on the bladder, like a laugh or a sneeze. Its placedthrough a small incision in the vagina and for women worried about leaking, its an effective option. Mostimportantly, the FDA did not ban bladder or vaginal mesh slings and it isendorsed by most female urology specialists and urogynecologists.
Also Check: Used Marine Fuel Bladder For Sale