Who Is A Candidate For Treatment With Sacral Neuromodulation
InterStim® therapy is approved for usage by Medicare in Australia in people older than 18 years of age whose symptoms have not responded to medical and conservative treatments over at least 12 months due to:
- Overactive bladder due to detrusor overactivity or
- Paradoxically also in patients with urinary retention which is not due to a blockage in the urinary tract
In Australia InterStim® therapy is also approved for use in some patients with refractory faecal incontinence which has not responded to other treatments.
Dont Miss: Tea Tree Oil Urinary Tract Infection
Will Interstim Treatment Work For Me
A simple in-office procedure initiates an evaluation to determine in as few as 3-7 days whether an implanted InterStim® system is likely to provide long-term control over symptoms.
Your doctor will discuss the evaluation procedure with you and the options for using either a temporary lead or long-term lead for the evaluation. You and your doctor will decide together whether your evaluation was successful. The evaluation is considered a success if you experience a significant reduction in your symptoms.
For example, your evaluation may be considered a success if you went to the bathroom 20 times per day before the evaluation and went 10 or fewer times per day during the evaluation.
How Interstim Therapy Works
Your sacral nerves control and regulate your bladder. If theyre damaged, this can lead to incontinence, because the signals between your brain and your bladder can get mixed up. If your brain receives a signal at the wrong time, it can lead to an unneeded urge to urinate. The FDA-approved InterStim system aims to improve communication along the sacral nerves by introducing electrical stimulation.
Nerve damage can result from a number of underlying health conditions, such as diabetes, a nervous system disorder, a spinal injury, an infection, or childbirth.
Don’t Miss: High Grade Bladder Cancer Recurrence
Neuromodulation & Nerve Stimulation For Ic/bps
Neuromodulation is Step Four in the AUA Treatment Guidelines for the treatment of interstitial cystitis. It uses a mild electrical impulse on various nerves to help maintain nerve function. Two different forms of neuromodulation are available, Urgent PC® and Interstim® . Both devices are approved for urinary frequency, urgency, incontinence and overactive bladder. Neither is approved for interstitial cystitis however they are frequently suggested for symptom management, hence their inclusion in the AUA Guidelines.
What To Expect After The Device Is Implanted
Most patients will notice a slight pulling or tingling sensation, according to Medtronic, which manufactures the InterStim devices. These sensations should not be painful if they are, contact your doctor. Sudden movement can also cause a change in how the stimulation feels, because the device shifted in proximity to your sacral nerve. This doesnt affect the effectiveness of the stimulation has changed. After a few weeks, patients typically report they dont notice the sensation anymore.
The goal is for InterStim therapy to help patients return to their daily routines without worrying about bowel or urinary incontinence. A successful procedure should help patients be more confident of their ability to go through life, taking long walks, traveling or visiting a movie theater. Things that were difficult become possible, once patients are no longer worried about incontinence.
Also Check: Bladder Infection From Hot Tub
How Is It Diagnosed
Thereâs no test for interstitial cystitis. If you go to your doctor complaining about bladder pain along with frequency and the urgency to pee, the next step is to rule out what else it could be.
Both men and women would first need to rule out urinary tract infections, bladder cancer, sexually transmitted diseases, and kidney stones.
In women, endometriosis is another possibility. For men, IC can be mistaken for an inflamed prostate or chronic pelvic pain syndrome.
These tests can rule out other conditions:
- Urinalysis and urine culture. Youâll be asked to pee in a cup. Itâll be sent to a lab to check for infection.
- Postvoid residual urine volume. Using an ultrasound, this test measures the amount of pee that remains in your bladder after you go to the bathroom.
- Cystoscopy. A thin tube with a camera is used to see the inside of the bladder and urethra. This is usually done only if there is blood in your pee or if treatment doesnât help.
- Bladder and urethra biopsy. A small piece of tissue is taken and tested. This is usually done during cystoscopy.
- Bladder stretching. Your bladder is filled with liquid or gas to stretch it out. Youâll be asleep under anesthesia. Sometimes this is also used as a treatment. This is done with a cystoscopy.
- Prostate fluid culture . Your doctor will need to press on your prostate and milk a sample to test. This is not commonly done.
Inserting The Interstim Therapy Device
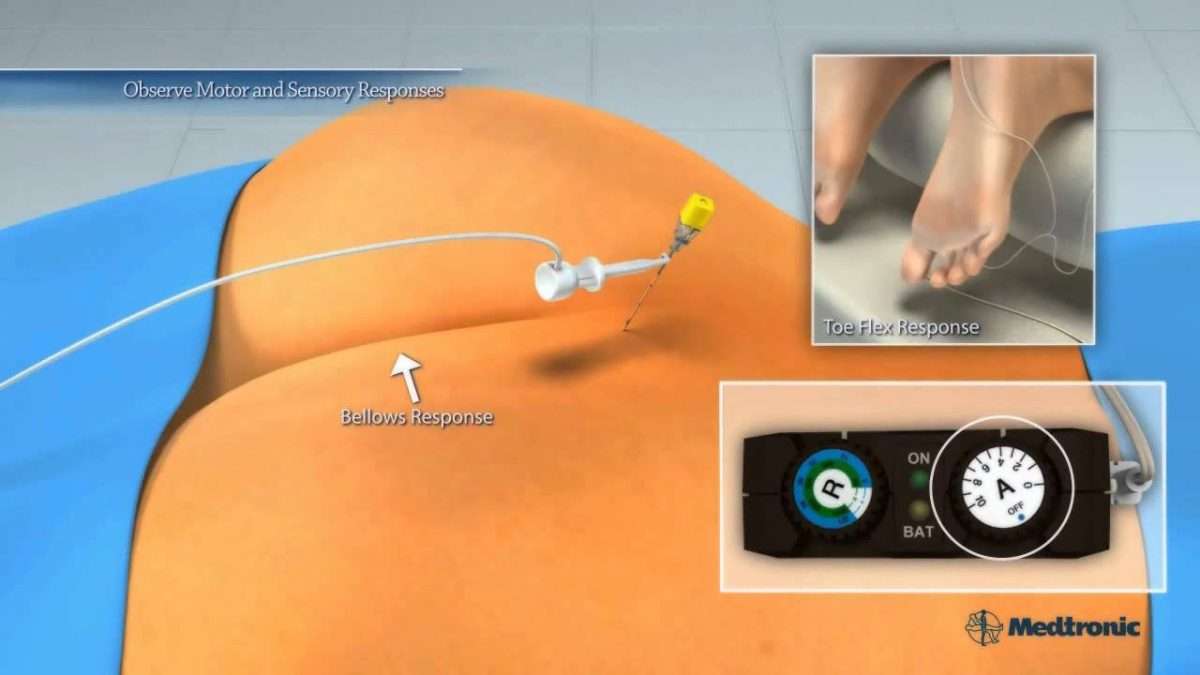
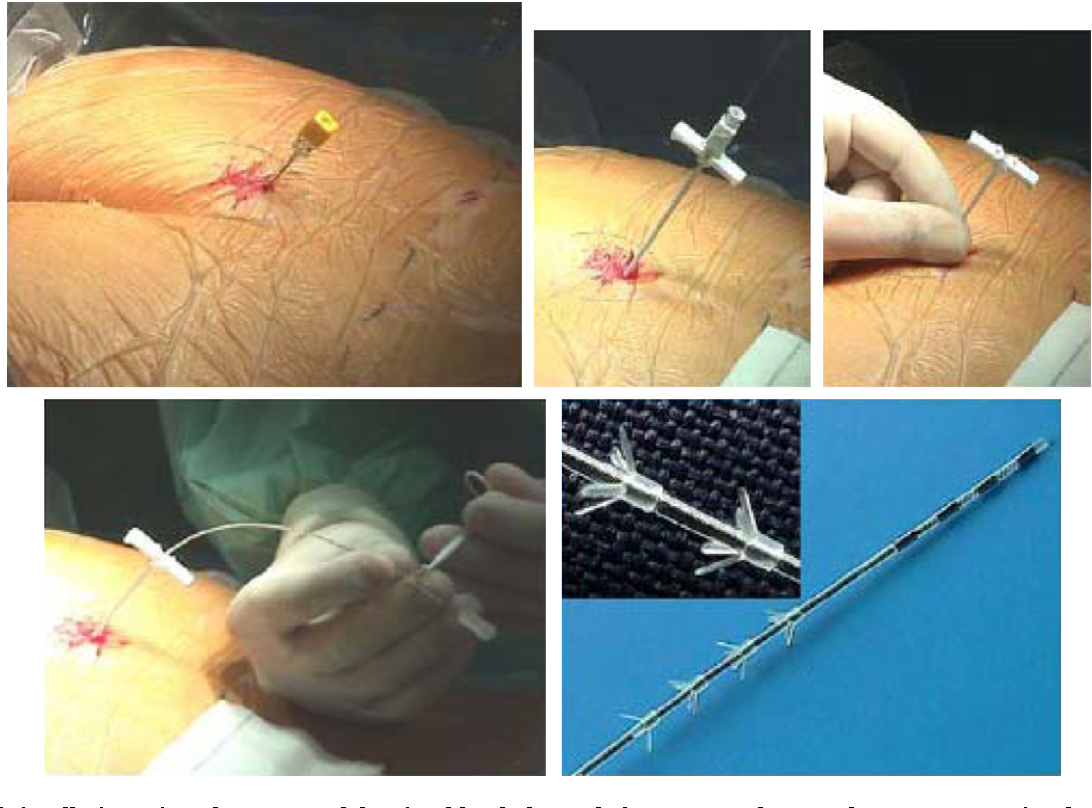
Since nerve stimulation is not an answer for everyone, there is a test trial period before the InterStim device is implanted. Doctors perform the test phase procedure in an operating room or a medical office. The doctor will numb a small area and insert a thin, flexible needle near the tailbone. This needle will be attached to a wire placed near the sacral nerves.
A small amount of electrical stimulation will test the patients sensation to find the best placement. A person can expect to feel a comfortable vibration, pulsing or tingling in the area of the vagina or rectum.
Once the doctor has located the optimal location, the temporary testing wire will be secured and attached to an external battery, which can be placed on the patients belt. The patient will have a remote to adjust the level of stimulation. This allows each patient to tailor the device to meet his or her needs.
The testing period will take between 1-3 weeks. During this time the doctor will ask the patient to complete a bladder diary to track daily urinary habits.
If there is improvement in the urinary or fecal symptoms, the second stage of the procedure will be performed to implant the permanent battery in the upper part of the buttock. The battery is similar to a heart pacemaker.
With both procedures, the patient will be able to go home the same day, but will need a driver.
You May Like: What Can You Take For Bladder Pain
Benefits Of Interstim Therapy
InterStim therapy is a simple, outpatient surgical procedure that is shown to give life-changing and long-lasting relief for symptoms of overactive bladder. InterStim therapy can be tried externally before receiving the implanted device. Your doctor can place a temporary external nerve stimulating device over the sacral nerves in the upper buttocks to perform a peripheral nerve evaluation and determine if this procedure is right for you. Over the course of a few days, your symptoms may improve by 50% or more, which indicates you are a candidate for InterStim therapy.
Bladder Pacemaker Restores Urinary Control
MEDIA CONTACTS: Ruthann Richter at Stanford, 723-6911, or Diana Marszalek at UCSF, 476-2557
EDITORS, REPORTERS PLEASE NOTE: A diagram with caption is attached.
FOR IMMEDIATE RELEASE
BLADDER PACEMAKER RESTORES URINARY CONTROL
Just as a cardiac pacemaker helps maintain a steady heartbeat, a new bladder pacemaker helps men and women with debilitating bladder problems regain control of this vital function, according to UCSF Stanford Health Care physicians, who pioneered the technology.
The implantable bladder pacemaker delivers a painless electrical stimulus to the nerve fibers that regulate the muscle of the bladder. This enables patients to control urine storage and release, said Dr. Emil Tanagho, a UCSF professor of urology whose early work with paraplegics and quadriplegics led to the development of the device.
He and Dr. Rodney Anderson, a Stanford professor of urology, are among the three physicians in California and the only two in Northern California who are currently implanting the device. The Food and Drug Administration approved the device in September 1997.
Tanagho said the new pacemaker may benefit patients suffering from urge incontinence, the inability to control the strong, sudden urge to urinate. It could also help people with severe bladder problems associated with multiple sclerosis, Parkinsons disease, interstitial cystitis or pelvic pain produced by overactive pelvic muscles, he said.
Implanted under skin
You May Like: How Common Are Bladder Infections
You May Like: What Medication Is Good For Overactive Bladder
The Process For Getting An Implant
The InterStim system consists of a neurostimulator and an electrode both of which are implanted in the lower back with small incisions and a wireless remote control. The neurostimulator sends the signals to the electrode, and the electrode stimulates the sacral nerves. The external wireless device allows you to adjust the signal level and turn the system on and off.
Youll be placed under anesthesia for your procedure, which is performed on an outpatient basis and typically takes about an hour. Unlike other surgical treatments, you can take InterStim for a test run to see if its right for you before making a long-term commitment. The initial evaluation period spans 3-14 days, in which time you can go about your normal routine and track your symptoms to see if there are noticeable improvements in your urge, control and frequency.
Medtronic Bladder Control Therapy Delivered By The Interstim System
THE RELIEF YOUVE BEEN WAITING FOR
When lifestyle changes and medications fail, Medtronic bladder control therapy delivered by the InterStim systems can help. This therapy is simple and discreet, and it delivers the kind of relief that lets you enjoy the activities you love without a second thought.
- Targets the nerves that control your bladder to help it function normally again
- 85% of people using the InterStim system achieved success in the rst year,4
- 3X greater improvements in quality of life compared to medications6
- Lets you see if it works before you and your doctor decide
- Allows you to get full-body MRI scans if you need them
- Hundreds of thousands of people have experienced relief with this safe, FDA-approved and minimally invasive therapy
THE CHOICE IS YOURS
- The recharge-free InterStim II system may be best for most people because its simple, convenient, and low maintenance.
- The rechargeable InterStim Micro system is smaller, lasts longer, and requires regular recharging sessions.
Don’t Miss: Malignant Neoplasm Of Overlapping Sites Of Bladder
How Does It Work
SNM uses mild electrical pulses to stimulate the sacral nerve located near the tailbone, which controls the bladder and other muscles that manage urinary function. A pulse generator device the size of a stopwatch, is surgically implanted into the buttocks. The pulses are delivered near the sacral nerve through an insulated wire called a lead, which is also implanted under the skin.
Stimulate Relief With Dr Paul Jo

If parts of your body are struggling to do their job, theyâre in need of a boost. The InterStim® system can give your urinary system that boost.
Dr. Paul Jo is experienced in treating incontinence and implementing the InterStim® system in patients. Dr. Jo has decades of experience researching and successfully treating urinary issues. Dr. Jo will analyze your condition and symptoms, determine the cause of your incontinence if not already known, and work with you to create a plan for success.
Also Check: How Do I Cure A Bladder Infection
Instructions For After The Procedure
You will have follow up appointments with your doctor to make sure the device is programmed in the best way to control your symptoms. You will receive a remote control device to control stimulation, and you may see the InterStim nurse or technician as needed to adjust settings for optimal benefit. This may take a few visits after the initial implant. Once the most appropriate settings are determined, you will need to be followed annually or as needed. Patients can expect up to 6 years of benefit before the device may need to be replaced due to battery depletion.
Percutaneous Tibial Nerve Stimulation
Percutaneous tibial nerve stimulation involves applying gentle electrical stimulation to the tibial nerve, which spans from the foot to the spine, improving bladder function and reducing how often you urinate and get up in the night with urgency incontinence.
Your NYU Langone urologist inserts a needle that is very fine, similar to those used in acupuncture, near the ankle. The needle is actually an electrode that delivers low-voltage electrical impulses through a nerve in the leg up to nerves that control the bladder muscle.
The procedure lasts 30 minutes and is performed once a week for 12 weeks in the doctors office. Follow-up treatments range from every few weeks to every few months.
Dont Miss: How To Train Bladder To Hold More Urine
Recommended Reading: Oxybutynin Dosage For Overactive Bladder
Interstim Bladder Control Therapy
If youre struggling with urinary control and the symptoms of overactive bladder, Medtronic Bladder Control Therapy may be able to help you.
Medtronic Bladder Control Therapy, also known as InterStim Therapy for Urinary Control, is used for the treatment of urinary retention and the symptoms of overactive bladder including urinary urge incontinence and significant symptoms of urgency-frequency. Generally, it is used for patients who have not responded to more conservative treatment options.
Whats The Process For Getting An Interstim Device
Stage 1, trial period and evaluation: InterStim therapy begins with a two-week trial period employing a wearable, external version of the device to determine if the treatment is likely to be effective for a patient. This is known as InterStim Stage 1.
Stage 2, surgery: If the trial produces positive results, the device is implanted in a patients lower back near the sacral nerves. Surgery is known as InterStim Stage 2.
Post-surgery: After surgery to implant an InterStim device, the doctor will program the devices electrical signals based on the results from the trial period.
Read Also: How Is Immunotherapy Administered For Bladder Cancer
Who Should Consider Medtronic Bladder Control Therapy Delivered By The Interstim System
Medtronic Bladder Control Therapy delivered by the InterStim System may be an option for patients with overactive bladder and non-obstructive urinary retention. Patients should consider Medtronic Bladder Control Therapy after they have tried other treatments such as fluid and diet modifications, medications and behavioral therapy and they have not worked successfully or could not be tolerated.*
Medtronic Bowel Control Therapy for the treatment of chronic bowel incontinence should be considered after patients with bowel control problems have tried other treatments such as medications and dietary modifications and they have not worked, or were not a candidate for these treatments.
The InterStim System does not treat every type of bladder control or bowel control problem. For more information, please visit: www.medtronic.com
See If It Works For You
Unlike other bladder control treatments, our therapy lets you try it first. Its called an evaluation like a test run, not a long-term commitment.
- Starts with a short, in-office procedure
- Go about most of your regular activities for 3-14 days
- Track your symptoms to see if they improve
- Talk with your doctor about your results and find out if its likely to help you
Complications can occur with the evaluation, including movement of the wire, technical problems with the device, and some temporary pain. Your doctor or nurse will show you how to use the system, and inform you of any activity restrictions and other precautions related to the evaluation.
You May Like: Does Coffee Cause Bladder Infections
What Bladder Control Problems Are Treatable
InterStim therapy has been shown to help patients alleviate symptoms associated with the following bladder control issues:
- Urge Incontinence: Causes a sudden urge to urinate. Urge incontinence is also referred to as an overactive bladder and may occur due to a bladder infection, bladder cancer, nervous system diseases, and other health conditions.
- Urinary Retention: Makes it difficult for an individual to empty the bladder. A person who is dealing with urinary retention may be unable to start urinating. Or, a person who is suffering from urinary retention may begin urinating but is unable to fully empty his or her bladder.
- Frequent Urination/Frequent Urge to Urinate: Occurs when a person passes only small amounts of urine, urinates frequently, and/or cannot fully empty his or her bladder.
Complication Rates By Type Of Sling
Before undergoing bladder sling surgery, women should ask their doctors about the technique they plan to use. Complication rates may vary depending on the type of mesh sling and technique.
A 2010 study of bladder sling procedures by Z. Chen and colleagues published in Urologia analyzed the outcomes of 187 women who received bladder slings to treat stress urinary incontinence. Authors found that transobturator vaginal tape inside-out and transobturator vaginal tape out-inside are simpler techniques with fewer complications compared to tension-free vaginal tape .
Women who used TVT had an average hospital stay of five days versus about two days for the TOT group.
The complication rate in the study was:
- 15.6 percent for tension-free vaginal tape
- 9.20 percent for transobturator vaginal tape inside-out
- 8.90 percent for transobturator vaginal tape out-inside
Complications from the procedures included discomfort with urinating, bleeding outside blood vessels and dysfunction of lower limbs. TVT was the only procedure associated with bladder perforation. Despite the complication rate, doctors found the slings safe.
The three tension-free urethral suspension techniques have similar efficacy, all of them are safe and effective procedures for the treatment of female SUI, authors wrote.
Don’t Miss: Questions To Ask Doctor About Bladder Cancer
Detection Of Cytokines Present In The Urine Of Ic/bps Patients With Or Without Hunner Lesions
Urine cytokines in the present study were detected using the mesoscale discovery U-plex assay: a highly sensitive electrochemiluminescence plate assay. Nine cytokines were selected for testing based on previous literature and their roles in the immune system: IFN-γ, TNF-α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-12p70, and IL-13.
IL-6 is a key cytokine involved in B cell activation and the results from the cytokine assay indicated that patients with IC/BPS-HL, when compared to their age and sex matched IC/BPS-NHL counterparts, displayed significantly higher average urinary concentrations of IL-6 . A significant difference in average urinary IL-6 concentrations was not observed between the IC/BPS-HL population and UC population .
Urinary Cytokine concentrations in patients with Interstitial Cystitis/Bladder Pain Syndrome , and unaffected controls . Nine cytokines -1β, IL-2, IL-4, IL-6, IL-8, IL-12p70, IL-13, and Tumor Necrosis Factor-α) were assessed using a MSD U-plex 10 spot assay . Individual and average cytokine concentrations for A IL-6, B TNF-α, C IL-12p70, and D IL-13 from patients with Interstitial Cystitis/Bladder Pain Syndrome without Hunner Lesions , patients with Interstitial Cystitis/Bladder Pain Syndrome without Hunner Lesions , and UC research participants. Data are expressed as mean±SEM
Dont Miss: Can A Bladder Infection Cause Bleeding