Checkpoint Inhibitors And Biomarkers
Checkpoint inhibitors and biomarkers, Dan will Im sure comment more about this. Its not clear that the markers are completely reliable when it comes to identifying PD1 or PD-L1 because many patients in spite of having negative PD-1 or PD-L1 staining still respond to some of our checkpoint inhibitors. Theres a lot of PD-L1 and PD-1 monoclonal antibodies out there so while a lot of this is a research standpoint and companion assays are approved, they are still not as predictive for response as we would like them to what we would like them to be.
Who Is A Good Candidate For Immunotherapy
Whether you may be a candidate for immunotherapy depends on the specific type and stage of your cancer, biomarkers that your cancer expresses, and whether current cancer treatment guidelines and data support immunotherapy for certain situations.
You may be a candidate for immunotherapy if:
- Genomic testing reveals biomarkers that are positive for PD-L1 expression, high microsatellite instability or high tumor mutational burden.
- You have advanced cancer. Generally, if youve exhausted your options for conventional treatment, you may be accepted into a clinical trial studying the effectiveness of an immunotherapy drug on your cancer type or on the genetic markers identified in its DNA.
- You have non-small cell lung cancer, especially if its metastatic or at an advanced stage. Genomic testing is now part of the guidelines for this type of cancer. Studies show that patients with advanced non-small cell lung cancer who respond to immunotherapy are living longer than those who didnt get access to immunotherapy. Some have been on maintenance doses for a long period of time.
Immunotherapy may be used in some cases to reduce the risk of relapse in those who have had a cure of their non metastatic cancer. This is more common in lung cancer and in some melanomas.
Depending on the type of cancer, immunotherapy may also be used in conjunction with chemotherapy.
What Are The Risks
Immunotherapy holds a lot of promise as a cancer treatment. Still, it can cause some problems.
You might have a bad reaction. The area where the medication goes into your body could hurt, itch, swell, turn red, or get sore.
There are side effects. Some types of immunotherapy rev up your immune system and make you feel like you have the flu, complete with fever, chills, and fatigue. Others could cause problems like swelling, weight gain from extra fluids, heart palpitations, a stuffy head, and diarrhea. Most of the time, these ease up after your first treatment.
It can harm organs and systems. Some of these drugs can cause your immune system to attack organs like your heart, liver, lungs, kidneys, or intestines.
It isnât a quick fix. In some cases, immunotherapy takes longer to work than other treatments. Your cancer may not go away quickly.
It doesnât work for everyone. Right now, immunotherapy works for less than half the people who try it. Many people only have a partial response. This means your tumor could stop growing or get smaller, but it doesnât go away. Doctors arenât sure yet why immunotherapy helps only some people.
Your body could get used to it. Over time, immunotherapy may stop having an effect on your cancer cells. This means that even if it works at first, your tumor could start to grow again.
Show Sources
Also Check: What Is The Treatment For Thickening Of The Bladder Wall
What Are The Benefits Of Immunotherapy
Key benefits of immunotherapy over conventional therapies, such as chemotherapy and radiation, include:
- Fewer immediate and long-term side effects
- The ability to continue treatment on a long-term basis while maintaining good quality of life
Conventional therapies may cure some cancers, but they may also cause difficult, long-term side effects, like peripheral neuropathy, heart problems, surgical complications, lung damage, hormone dysfunction and memory and cognition problems. Eventually, standard therapies may also compromise or overpower the immune system.
Cancer immunotherapy, on the other hand, may have fewer immediate and long-term side effects. The most common immunotherapy side effects patients may experience while receiving treatment include:
- Autoimmune response
If you experience a side effect from immunotherapy, we may be able to treat it directly, or we may delay your next treatment to allow you some time to recover. Supportive care therapies may also help you manage side effects. Occasionally, steroids may be used to suppress the reaction. If the reaction involves a severe autoimmune response, you may have to discontinue immunotherapy.
Bcg Immunotherapy In Nmbic
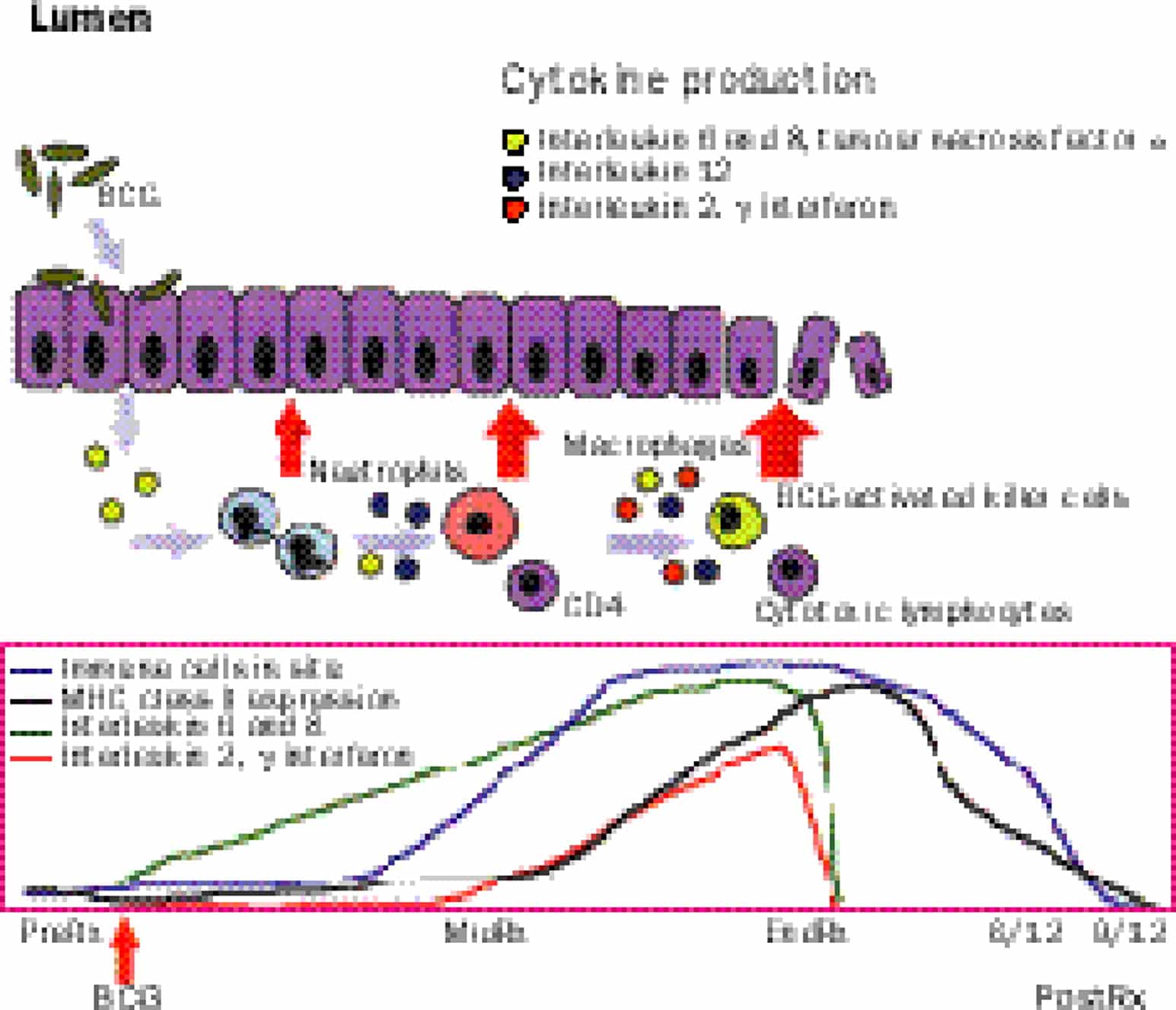
So of course BCG is the big dog when it comes to immunotherapy of almost every cancer. Its the model. It was one of the original ones that out there, where youre stimulating your own immune system to reject the cancer. The interesting thing is BCG is also being used for melanoma, directly injecting BCG into melanoma has caused many remissions. But BCG immunotherapy everybody knows is our standard of care. It can reduce recurrent/progression. It has to be given at least one year, and probably up to three months for high-risk non-muscle invasive bladder cancer and overall is superior to intravesical chemotherapy. So we set the stage for talking about basic concepts in immunotherapy knowing that we as urologists who would be using BCG extensively since the late 1980s and early 1990s. There surely should be the thought leaders in a lot of this area of immunotherapy.
Read Also: Does Menopause Cause Bladder Leakage
What Are The Top Benefits Of Bcg Treatment For Bladder Cancer
If the doctor decides that this treatment option is right for you, there are several significant benefits to keep in mind. They include:
- This is a relatively non-invasive treatment process.
- It can help you save your bladder and prevent the spread of cancer cells to other parts of your body.
- It does not require any other medications to work effectively.
- It stimulates the immune system to attack cancer cells without attacking healthy cells.
What Are Contraindications To The Administration Of Bcg
Literature review and analysis
Instillation of BCG in the presence of gross hematuria can result in systemic absorption and toxicity from BCG. Thus, BCG should not be instilled in the presence of gross hematuria or active urinary infection. Treatment of ongoing urinary tract infections prior to BCG instillation may reduce toxicity. In a study in which patients with high-risk NMIBC received induction intravesical BCG, 61/243 had significant bacteriuria in voided urine prior to starting therapy. In this study, asymptomatic bacteriuria did not appear to increase side effects or risk of BCG toxicity and had no impact on recurrence rates in infected patients . Although BCG has been cited as contraindicated for immunologically compromised patients with bladder cancer, a retrospective study reported on 45 immunosuppressed high risk NMIBC patients treated with intravesical BCG. Of these patients, 12 had functioning organ transplants, 23 were undergoing chemotherapy for unrelated cancers, and 10 were taking steroids for autoimmune or related diseases. Although this study was conducted in a small patient population, these results suggest that BCG can be safely administered to select patients who are immunosuppressed. However, efficacy may be limited, as individuals receiving immunosuppression following organ transplantation were less likely to respond .
Consensus recommendations
Don’t Miss: Does Amoxicillin Treat Bladder Infections
Bladder Cancer And Immunotherapy Session Transcript
Tamron Hall: Welcome back if you’re just joining us right now, it’s my pleasure to welcome you to the Cancer Research Institute first ever virtual immunotherapy patient summit, and our breakout session on bladder cancer immunotherapy. I’m delighted to introduce to you Dr. Terence Friedlander, who is here to share the latest on bladder cancer immunotherapy, and answer your questions. Dr. Friedlander is chief of hematology oncology at Zuckerberg, San Francisco General, and an associate professor of medicine at the University of California, San Francisco, Helen Diller Family Comprehensive Cancer Center. Brian Brewer from the cancer research Institute, we’ll be sharing your questions with Dr Friedlander. So please be sure to put them in the Q and A box. Dr. Friedlander, thank you so much for sharing your expertise with us today.
And then as I’ve mentioned already, there are a lot of novel agents under investigation. There’s new types of viral therapies being given into the bladder to try and sort of replicate what BCG does. Giving immune therapy before or after surgery to remove the bladder, there’s studies evaluating that. And obviously I’ve talked about some of these studies in metastatic disease. So there’s really a broad development of these agents completely across the spectrum of bladder cancer. And that’s really exciting. So with that, I think we’re going to move on to some of the questions and I’m here with Brian Brewer. Who’s going to, I think, take it from here.
Substantial Improvement In Survival
Dr. Powles and his colleagues enrolled 700 people with locally advanced or metastatic bladder cancer in the international JAVELIN Bladder 100 study, which was funded by Pfizer, the drug’s manufacturer.
All trial participants had already received chemotherapywith either cisplatin and gemcitabine or carboplatin and gemcitabine, if their health did not allow them to receive cisplatinand their disease had not worsened during chemotherapy.
Participants were then randomly assigned to receive either maintenance treatment with avelumab plus supportive care or supportive care alone. People in the maintenance group received infusions of avelumab every 2 weeks until their cancer started growing again or they left the study for other reasons. Supportive care for both groups included pain management, nutritional support, and treatment of infections.
People in the supportive care group whose cancer got worse did not receive avelumab as part of the trial. However, they could receive it or any other immunotherapy drug after leaving the study.
Maintenance treatment with avelumab after chemotherapy turned out to have substantial benefits. The median overall survival for people who received maintenance avelumab was more than 21 months, compared with about 14 months for people who received only supportive care until their cancer got worse.
Read Also: What Medications Can Cause Overactive Bladder
Intratumoral Morphologic Heterogeneity Is Associated With Intratumoral Immunologic Heterogeneity
Intratumoral genetic heterogeneity can result in a large subclonal mutational burden that may facilitate the selection of tumor subclones resistant to immune checkpoint blockade. We, therefore, hypothesized that morphologic heterogeneity could be a predictive biomarker of poor response to anti-PD-1/PDL-1 therapies in bladder cancer patients. To test this hypothesis, we performed a detailed histologic review of bladder tumors collected from 29 patients with locally advanced or metastatic bladder cancer that were treated with the anti-PD-L1 antibody atezolizumab on a therapeutic protocol . This analysis was restricted to performing histopathologic review of hematoxylin and eosin-stained slides from the tumors included in this study and correlation with response to atezolizumab therapy and did not include use of any genomic data from that study. Of the 9 patients who experienced durable clinical benefit, histopathologic evaluation revealed overwhelming tumor morphologic monotony and a lack of morphologic heterogeneity in 7 patients . In contrast, 16 of 20 patients who derived no clinical benefit from atezolizumab had tumors with mixed histology .
Fig. 4: Distinct immune response gene signatures in paired UC and SqD samples.
Principles Of Cancer Immunotherapy Continued
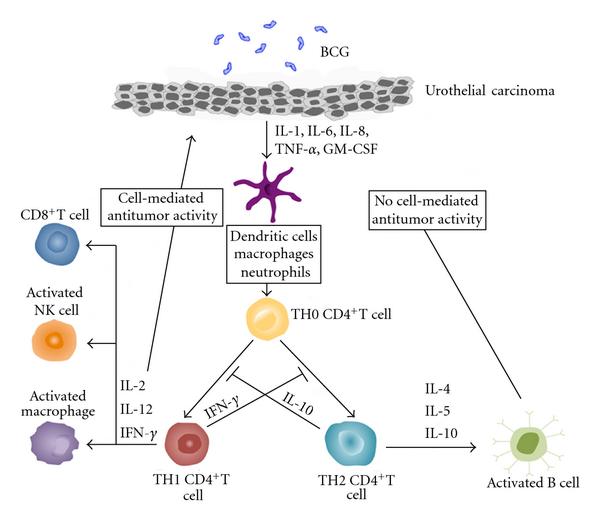
So when you look at immunotherapy we have two general classes, passive immunotherapy and active immunotherapy. For urologic tumors, passive immunotherapy doesnt do us a lot of good because for passive immunotherapy you have to continually administer these medications, and as the tumors grow you cant really keep up with the growth of the tumor and really try to clear it. But there are monoclonal antibodies out there for other diseases which are effective. However, for things like bladder cancer and kidney cancer they tend not to be as effective. However active immunotherapy, where we kick our own T cells into high performance to identify tumors is really some of the most effective approaches that we have. So remember that antigens on the tumor cell need to be presented to T cells, and antigen-presenting cells, and I might have said that backwards a minute ago. The macrophages eat up these tumors. They then process the antigens, stick them outside and tell the T cells please seek these tumors out and destroy them. So thats this antigen presentation concept. Dendritic cells, of course Dendreon that name sounds kind of familiar, is a way of taking antigen-presenting cells, putting them back in the body and its those APCs in Dendreons Provenge for example, you put it back into the patient. Those dendritic cells identify prostate cells. By identifying those cells, they then tell the T cells go after this particular tumor.
Also Check: Do Bladder Infections Cause Headaches
Basic Concepts In Bladder Cancer Immunotherapy
Posted by Leonard G. Gomella, MD, FACS | Mar 2018
Leonard G. Gomella, MD, FACS, presented Basic Concepts in Bladder Cancer Immunotherapy at the 2nd Annual International Bladder Cancer Update on January 27, 2018 in Beaver Creek, Colorado
How to cite: Gomella, Leonard G. Basic Concepts in Bladder Cancer Immunotherapy January 27, 2018. Accessed Nov 2022. https://grandroundsinurology.com/Basic-Concepts-in-Bladder-Cancer-Immunotherapy/
Bladder Cancer Consensus Task Force
The Task Force consisted of 17 participants, including 8 medical oncologists, 7 urologists, 1 nurse, and 1 patient representative . The urologists were chosen for their experience in the development and evaluation of best practice guidelines for the use and optimization of BCG therapy, and all members were experts in the management of the spectrum of urothelial cancer. The medical oncologists were experienced in the management of advanced bladder cancer with both chemotherapy and immunological therapy, including participation in clinical trials of immune checkpoint inhibitors. Additional participants were experts in addressing issues of barriers in access to appropriate use of immunotherapy. A list of the Task Force pre-meeting survey questions and responses is available in Additional file .
Read Also: Is Bladder Infection Same As Urinary Tract Infection
What Is The Recommended Initial And Maintenance Dose Of Bcg
Literature review and analysis
Based on clinical trials and clinical experience, the initial course should be 1 vial of BCG usually containing approximately 5 × 108 or more CFU weekly for 6 weeks . This is accepted by the AUA, EUA, and the IBCG .
Increasing side effects may be reduced by serial reductions in BCG dose most recommended dose reductions are at one-third, one-tenth, one-thirtieth, and one-one hundredth . Randomized clinical trials have reported conflicting results regarding the efficacy and improved safety of dose reduction. The highly cited randomized trial by Oddens et al. showed efficacy in the following order: full dose for 3 years, one-third dose for 3 years, full dose for one year, and lastly one-third dose for one year .
Consensus recommendations
The Task Force recommended full doses for induction and the dose reduction during maintenance if necessary based on side effects, which is based on Level A evidence. The Task Force did concede that during times of BCG shortage, as has happened in recent times, it is acceptable to start induction with one-third dose if this allows a vial of BCG to be split among 3 patients to allow more patients to receive BCG than if this were not done.
What To Expect During Bcg Treatment
First, make sure you havent had any fluids for four hours before the treatment. Right before you go into the treatment room your doctor or nurse will have you empty your bladder.
Youll lie on your back, and the medical professional will insert a catheter into your urethra and into your bladder, likely using some local numbing, and use this tube to infuse the treatment.
Once the treatment is infused, your doctor or nurse will remove the catheter. Theyll have you lie on your back, each side, and your stomach for 15 minutes each. The BCG mycobacteria needs to touch the bladder cancer cells to activate the immune system. Youll then be free to go but will need to hold off on peeing for another hour.
Verywell / Alex Dos Diaz
For at least six hours after your infusion, youll need to disinfect your pee to ensure none of the mycobacteria spread to anyone else. Pour an equal amount of bleach into the toilet after you pee and let it sit for 15 minutes before flushing.
You will likely need multiple BCG treatments. They may be given weekly for a few weeks, then less often for months or years to prevent cancer from coming back.
Don’t Miss: Device To Stop Bladder Leakage
How Can Patient Support During The Management Of Nmibc Enhance Access To Appropriate Management
Literature review and analysis
Approximately 50% of patients with newly diagnosed NMIBC do not receive appropriate therapy with intravesical BCG. Reasons for this are myriad, including reluctance on the part of the patient and the physician, lack of appreciation of the potential benefit, and access to appropriate facilities that can administer BCG. Patient navigation approaches, or support programs developed to help guide patients through the care system, appear to greatly improve the latter, providing timely access to appropriate care . Additionally, the Urologic Diseases in America Project has documented the underuse of guideline-recommended care in NMIBC as well as in invasive disease . It is proposed that implementing patient navigation programs may reduce the time from diagnosis to treatment of NMIBC and could increase the likelihood of actually undergoing intravesical therapy in eligible survivors. Additionally guideline-appropriate care is likely to improve outcomes for most categories of early bladder cancer. This proposal is extrapolated from a large meta-analysis of patients with abnormal breast, cervical, colorectal, or prostate cancer screening outcomes and the role of patient navigators to facilitate timely cancer care .
Consensus recommendations
What Is The Role Of Maintenance Bcg
Literature review and analysis
All guidelines recommend induction and maintenance BCG of 13 years for high risk patients with a risk reduction in terms of recurrence . However, the ICUD guidelines only include maintenance BCG for carcinoma in situ, not for Ta high grade tumors . This differs from the recommendations of AUA, EUA, and IBCG. As reviewed in previous sections, BCG induction and maintenance has been shown to be beneficial in patients with high risk and intermediate risk groups utilizing the SWOG schedule . Modifications in terms of reduction in dose or in the number of doses per session of maintenance have not been shown as beneficial . Again, an improved definition of the patient subgroups who would benefit continues to be a topic of active clinical research. The report of EORTC 98013 suggests that 1 year of maintenance utilizing the SWOG schedule is sufficient for intermediate risk patients . However, recurrence directly correlated with the duration of maintenance, with 3-year maintenance resulting in fewer recurrences in each dose group.
Consensus recommendations
The members of the Task Force had different opinions on this issue. However, it was agreed upon that all high risk patients should receive maintenance therapy for 3 years, while intermediate risk patients should receive maintenance therapy for at least 1 year based on Level A evidence.
You May Like: Can Lower Back Pain Cause Bladder Problems