What Should I Do With This Leaflet
Thank you for taking the trouble to read this patient information leaflet. If you wish to sign it and retain a copy for your own records, please do so below.
If you would like a copy of this leaflet to be filed in your hospital records for future reference, please let your urologist or specialist nurse know. If you do, however, decide to proceed with the scheduled procedure, you will be asked to sign a separate consent form which will be filed in your hospital notes and you will, in addition, be provided with a copy of the form if you wish.
I have read this patient information leaflet and I accept the information it provides.
Signatureâ¦â¦â¦â¦â¦â¦â¦â¦â¦â¦â¦â¦.â¦â¦â¦â¦â¦Dateâ¦â¦â¦â¦â¦.â¦â¦â¦â¦â¦â¦â¦.
How Is Mitomycin Given
Mitomycin is given as an infusion into a vein. A healthcare provider will give you mitomycin.
Tell your caregivers if you feel any burning, pain, or swelling around the IV needle when mitomycin is injected.
Some people receiving mitomycin have developed ulcers on the skin where an injection was given, or on other areas of body. Skin changes may occur several weeks or months after a mitomycin injection.
Mitomycin affects your immune system. You may get infections more easily. You will need frequent medical tests, and your next dose may be delayed based on the results.
Mitomycin can have long lasting effects on your body. You may also need medical tests for a short time after your last dose.
Mitomycin Dosage And Administration
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Mitomycin should be given intravenously only, using care to avoid extravasation of the compound. If extravasation occurs, cellulitis, ulceration, and slough may result.
Each vial contains Mitomycin, USP 5 mg and mannitol 10 mg. To administer, add Sterile Water for Injection, 10 mL. Shake to dissolve. If product does not dissolve immediately, allow to stand at room temperature until solution is obtained.
After full hematological recovery from any previous chemotherapy, the following dosage schedule may be used at 6 to 8 week intervals:
20 mg/m 2 intravenously as a single dose via a functioning intravenous catheter.
Because of cumulative myelosuppression, patients should be fully reevaluated after each course of Mitomycin, and the dose reduced if the patient has experienced any toxicities. Doses greater than 20 mg/m 2 have not been shown to be more effective, and are more toxic than lower doses.
The following schedule is suggested as a guide to dosage adjustment:
| < 25,000 | 50% |
No repeat dosage should be given until leukocyte count has returned to 4000/mm 3 and a platelet count to 100,000/mm 3.
Recommended Reading: How Do You Get Rid Of Overactive Bladder
Possible Side Effects Of Mitomycin Given Into The Bladder
- Mitomycin can cause skin irritation if it comes into contact with the skin. Washing the area with soap and water after urinating can reduce this risk.
- Some patients will develop a bladder infection after this procedure. If you experience an urgency to urinate, burning or pain with urination, blood in the urine or fever, notify your healthcare team right away.
- Intravesicular chemotherapy may cause burning with urination, cramps and diarrhea.
- In some rare cases, mitomycin can cause a decrease in your blood counts. This can include white blood cells , platelets and red blood cells. This is more likely to occur when the medication is given after surgery and there is injury in the bladder , which can allow the medication to be absorbed into the blood stream. However, it can occur with any instillation.
Significantly Lower Recurrence Risk Seen
The recurrence risk in the entire cohort was significantly lower at 27% in the immediate instillation group compared to 36% in the delayed instillation group . Further, the difference in time to recurrence after 3 years of follow-up significantly favored an immediate instillation, with 34% reduction in the relative risk of recurrence compared to delayed instillation. The 3-year cancer progression rate was lower with immediate instillation compared to delayed instillation . However, the trial was not powered or designed to evaluate the risk of progression.
When analyzing each risk group separately, no difference was noted in the risk of recurrence in the LR group from immediate versus delayed instillation . However, immediate instillation significantly reduced the risk of recurrence in both the IR and HR group .
Read: ADT plus salvage RT lowers PCa metastases, raises survival
Adverse effects were recorded in 258 of 1,048 patients in the immediate instillation group and 257 of 1,195 patients in the delayed instillation group . Most common adverse effects were skin rash and irritative voiding symptoms . In six patients in the immediate instillation group, MMC extravasation was reported, which was managed conservatively.
It appears that for all NMIBC patients, an immediate single instillation of MMC within 24 hours after TURBT reduces the recurrence rate and prolongs time to recurrence, regardless of whether adjuvant MMC instillations were given.
Recommended Reading: How To Reduce Bladder Infection Pain
What Does The Procedure Involve
Your consultant urologist has referred you for treatment of your superficial bladder cancer. The treatment involves instillations of liquid chemotherapy into the bladder through a catheter once a week for six weeks. This is recommended for intermediate risk superficial cancer of the bladder. Superficial bladder cancer has the potential to recur. The aim of the treatment is to reduce the chances of recurrence over the next five years.
What Should I Expect Before The Procedure
You will be asked to come to the hospital once a week for six weeks. You should limit your fluid input for six hours before each treatment.
Your first treatment will take up to 90 minutes. On arrival in the clinic, you will be asked to pass urine which will be tested to ensure that you do not have an infection in the urine. If you do, your treatment will need to be postponed for one week while you are treated with antibiotics.
Please be sure to inform your urologist in advance of your surgery if you have any of the following:
- an artificial heart valve
- an artificial blood vessel graft
- a neurosurgical shunt
- any other implanted foreign body
- a prescription for warfarin, aspirin, rivaroxaban, dabigatran, apixaban, edoxaban or clopidogrel , ticagrelor or blood thinning medication
- a previous or current MRSA infection
- high risk of variant CJD
Recommended Reading: Does Diabetes Cause Bladder Problems
What Is The Evidence Base For This Information
This leaflet includes advice from consensus panels, the British Association of Urological Surgeons, the Department of Health and evidence based sources it is, therefore, a reflection of best practice in the UK. It is intended to supplement any advice you may already have been given by your urologist or nurse specialist as well as the surgical team at Addenbrooke’s. Alternative treatments are outlined below and can be discussed in more detail with your urologist or specialist nurse.
What Mitomycin Is Used For
It is used as palliative cancer therapy in:
- advanced metastatic stomach cancer
- advanced and/or metastatic breast cancer
- cancer of the respiratory tract
- advanced cancer of the pancreas
It is also used for the prevention of a relapse in the case of superficial urinary bladder cancer after the ablation of tissue through the urethra
You May Like: How To Relieve Bladder Spasms With Catheter
Before Taking This Medicine
You should not be treated with mitomycin if you are allergic to it, or if you have:
-
low levels of platelets in your blood
-
bone marrow suppression or
-
active bleeding or any risk of bleeding.
Tell your doctor if you have ever had:
-
kidney disease
Mitomycin may harm an unborn baby. Use effective birth control to prevent pregnancy, and tell your doctor if you become pregnant.
You should not breastfeed while using mitomycin.
Indications And Usage For Mitomycin
Mitomycin for Injection, USP is not recommended as single-agent, primary therapy. It has been shown to be useful in the therapy of disseminated adenocarcinoma of the stomach or pancreas in proven combinations with other approved chemotherapeutic agents and as palliative treatment when other modalities have failed. Mitomycin is not recommended to replace appropriate surgery and/or radiotherapy.
Also Check: Lemon Water For Bladder Infection
Mitomycin Coupons Copay Cards And Rebates
Mitomycin offers may be in the form of a printable coupon, rebate, savings or copay card, trial offer, or free samples.Some offers may be printed right from a website, others require registration, completing a questionnaire,or obtaining a sample from the doctor’s office.
There are currently no Manufacturer Promotions that we know about for this drug.
What Should I Expect When I Get Home
You should drink plenty of fluids for the few days after the treatment. Some patients find that, for a few days after mitomycin C treatment, a glass of cranberry juice daily eases any bladder symptoms cranberry juice, however, should not be used if you are taking warfarin.
If you think you have a urine infection , it is important to contact your GP and get treatment with antibiotics.
Because this treatment is put directly into the bladder and not into the blood stream, you will not experience the side effects often associated with other cancer drug treatments. You should also inform your specialist nurse if this happens.
You May Like: Can You Have Intercourse While Having A Bladder Infection
How To Take Mitomycin
Mitomycin is given directly into the bladder , through a catheter, and left in the bladder for 1-2 hours. The dosage and schedule is determined by your healthcare provider.
This drug is blue in color and may make your urine blue-green in color. This can last up to two days after each dose. It is not uncommon to have urinary frequency or painful urination for 24 hours after treatment. If this continues after 24 hours, call your doctor or nurse.
Patient Assistance & Copay Programs For Mitomycin
Patient assistance programs are usually sponsored by pharmaceutical companies and provide free ordiscounted medicines and copay programs to low income or uninsured and under-insured people who meet specific guidelines.Eligibility requirements vary for each program.
There are currently no Patient Assistance Programs that we know about for this drug.
Recommended Reading: What Are The Best Bladder Control Pads
Are There Any Side Effects
Most procedures have a potential for side effects. You should be reassured that, although all these complications are well recognised, the majority of patients do not suffer any problems after a urological procedure.
Please use the check boxes to tick off individual items when you are happy that they have been discussed to your satisfaction:
How Is Mitomycin Supplied
Mitomycin for Injection USP
NDC 72819-152-95 5 mg individually-boxed amber vial.
Storage: Store dry powder at 20° to 25°C , protected from light. Avoid excessive heat, over 40 °C . Protect reconstituted solution from light. Store solution under refrigeration 2° to 8 °C , discard after 14 days. If unrefrigerated, discard after 7 days.
Read Also: Over The Counter Bladder Infection Medicine
Are There Any Other Important Points
You should wash your hands and genitals after you have passed urine and it is advisable to bring a wash bag with you to hospital when you come for the treatment.
You are advised not to have sexual intercourse for at least 24 hours after the treatment as this can cause some discomfort.
If you are a smoker, we will encourage you to stop since smoking seems to encourage recurrence of bladder cancer.
Could One Strain Of Bcg Be Better Than Another
For non-muscle-invasive bladder cancer, use of the Connaught strain of bacillus Calmette-Guérin may be more effective at preventing recurrences than the Tice strain of BCG. BCG is an immunotherapy that is a weakened form of a bacterium that is instilled directly into the bladder through the urethra and exerts its anti-cancer effect by stimulating the bodys immune system to kill cancer cells. Different strains of BCG are available for use in the treatment of bladder cancer, and its possible that these different strains could differ in effectiveness.
To compare two strains of BCG that are commonly used in the United Statesand Europethe Connaught strain and the Tice strainresearchers in Europe conducted a Phase III clinical trial among 149 patients with early bladder cancer. Half the patients were treated with the Connaught strain and half were treated with the Tice strain.5
Five-year survival without a recurrence was 75 percent among patients treated with the Connaught strain of BCG and 46 percent among patients treated with the Tice strain. Side effects did not vary significantly by type of BCG.
These results suggest that the Connaught strain of BCG may be more effective than the Tice strain for the treatment of non-muscle-invasive bladder cancer.
References
Also Check: Do I Have A Bladder Problem
Medac Launches First Complete Mitomycin Instillation Kit
medac Pharma has launched the first complete Mitomycin instillation kit available for the treatment of non-muscle invasive bladder cancer . Each kit contains a closed instillation system, Mitomycin medac 20mg or 40mg vial, male and female catheter, clip and disposal bag.
This new advance in treatment of NMIBC is part of medacs Bladder Cancer Care programme. The kit has been designed to be easy to prepare and therefore speeds up the process of Mitomycin instillation therapy. Another benefit of the closed system is that it prevents cytotoxic contamination.1 Furthermore, Mitomycin medac is a new formulation which dissolves rapidly and also contains urea to reduce irritation to the patient.2
Mitomycin is indicated for intravesical administration to prevent relapse in adult patients with superficial urinary bladder carcinoma following transurethral resection. Available in 20mg and 40mg vials complete with instillation set, Mitomycin can be administered as both induction and maintenance therapy.
medac has also introduced the NMIBC toolbox an online, interactive aid for interpreting the EAU guidelines on NMIBC. This is available to download via the QR code.
Mitomycin medac complete instillation kit
References
- Lyvén B. Investigation of sealing properties of a system intended for safe mixing of drugs. SP Technical Research Institute of Sweden. January 2009.
Side Effects Requiring Immediate Medical Attention
Along with its needed effects, mitomycin may cause some unwanted effects. Although not all of these side effects may occur, if they do occur they may need medical attention.
Check with your doctor or nurse immediately if any of the following side effects occur while taking mitomycin:
More common
- difficult, burning, or painful urination
- lower back or side pain
- sores, ulcers, or white spots on lips or in mouth
- Fainting or loss of consciousness
- fast or irregular breathing
- lower abdominal or stomach pain
- swelling of the eyes or eyelids
- tightness in the chest
Also Check: Side Effects Of Bladder Cancer Chemotherapy
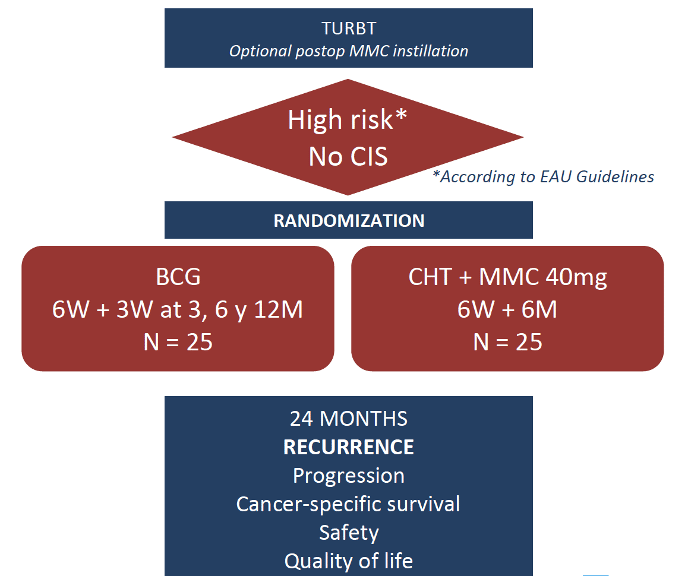
Eau : A Randomized Clinical Trial Of Intravesical Instillation Of Mitomycin
A total of 165 patients with NMIBC were randomly allocated to mitomycin C and mitomycin C plus Ara-C intravesical therapy group. As follows is a flow chart for the trial:
Intravesical therapy was performed once a week for six weeks. Outcomes assessed included recurrence-free survival, up-stage and -grade in recurrent tumor, toxicity, and influence of urine pH.There was no difference in clinicopathological features including grade, pT stage, and frequencies of recurrent and multiple tumors between the two groups. Kaplan-Meier survival curves showed that in the entire cohort recurrence-free survival in mitomycin C + Ara-C group was significantly longer compared to those in mitomycin C group. This benefit was also significant in patients with intermediate-risk disease , but not in high-risk patients :
- Recurrence-free survival in the mitomycin C plus Ara-C group was better than that in the mitomycin C group in patients with intermediate-risk NMIBC
- This randomized clinical trial suggests that intravesical therapy with mitomycin C and Ara-C is useful and safe for these patients
- Increasing urine pH by Ara-C instillation is speculated to be a main mechanism of increased anti-cancer effects of mitomycin C
Presented By: Yasuyoshi Miyata, MD, Ph.D., Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan
Comparison Of Intravesical Bacillus Calmetteguerin And Mitomycin C Administration For Nonmuscle Invasive Bladder Cancer: A Metaanalysis And Systematic Review
Copyright: ©Jianget al. This is an open access article distributed under theterms of CreativeCommons Attribution License.
This article is mentioned in:
Abstract
Introduction
Bladder cancer is the ninth most commonly occurringcancer globally. In total, 7080% of all bladder cancer patientsinitially present with superficial disease . Non-muscle invasivebladder cancers are aheterogeneous group of tumors that vary in terms of oncologicaloutcome .
Generally, the initial approach to managingnon-muscle invasive bladder cancer is cystoscopic observationfollowed by transurethral resection. The recurrence rate innon-muscle invasive bladder cancer is high following resection andthe disease can progress to muscle invasive cancer, which has apoor prognosis .
The present meta-analysis further investigated thebenefits of BCG and mitomycin C in the treatment of patients withsuperficial bladder cancer by comparing progression-free survival rates in patients treated with either mitomycin C or BCHfollowing transurethral resection.
Materials and methods
Search strategy
Data extraction
Quality assessment
Statistical analysis
Results
Literature search
Study characteristics
Table I.Study characteristics of the includedstudies. |
Table I.
Study characteristics of the includedstudies.
Table II.
5-year PFS rate
Sensitivity analysis
Recommended Reading: Ovary Pain When Bladder Is Full
How The Intravesicular Treatment Is Given
- You should limit your fluid intake starting the night prior to the procedure and have no fluids for 4 hours before. This is so you will be able to hold your urine in during the procedure for the full treatment time. In addition, the area receives more concentrated doses of the drug with less urine output during the procedure.
- If you take a diuretic , you will be told to not take this for at least 4 hours before the procedure.
- A urinary catheter is inserted into the bladder and any urine is drained.
- The mitomycin is given through the catheter, into the bladder. The catheter may be removed or clamped and remain in place based on your providers recommendation.
- You will need to hold the mitomycin in your bladder for 1-2 hours. You may need to change positions every 15 minutes to be sure the drug reaches all areas of the bladder. Do this by rolling on your side, back, other side and stomach.